Lecture 14 carbohydrates complete to be taught
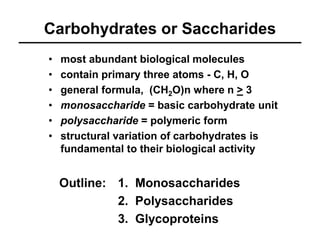
- 1. Carbohydrates or Saccharides • most abundant biological molecules • contain primary three atoms - C, H, O • general formula, (CH2O)n where n > 3 • monosaccharide = basic carbohydrate unit • polysaccharide = polymeric form • structural variation of carbohydrates is fundamental to their biological activity 1. Monosaccharides 2. Polysaccharides 3. Glycoproteins Outline:
- 2. Monosaccharides - definitions • Monosaccharides are aldehyde or ketone derivatives of straight-chain polyhydroxy alcohols containing at least 3 carbon atoms. • Aldose = carbohydrate containing an aldehyde • Ketose = carbohydrate containing a ketone Carbon atoms in alcohol Name 3 triose 4 tetrose 5 pentose 6 hexose 7 heptose . .
- 3. Monosaccharides - 3, 4, 5 carbon aldoses H Aldotriose Aldotetroses CHO C OH H C OH H C OH CH2OH H CHO C OH HO C H H C OH CH2OH CHO HO C H H C OH H C OH CH2OH D-Ribose (Rib) D-Arabinose (Ara) D-Xylose (Xyl) H CHO C OH H C OH CH2OH D-Erythrose HO CHO C H H C OH CH2OH D-Threose H CHO C OH CH2OH D-Glyceraldehyde Aldopentoses Fischer projections
- 4. Monosaccharides - 6 carbon aldoses H CHO C OH HO C H H C OH H C OH CH2OH CHO HO C H HO C H H C OH H C OH CH2OH CHO H C OH HO C H HO C H H C OH CH2OH CHO H C OH H C OH H C OH H C OH CH2OH D-Allose D-Glucose (Glc) D-Mannose (Man) D-Galactose (Gal) Aldohexoses
- 5. Monosaccharides - ketoses Ketotriose Ketotetrose CH2OH C O H C OH CH2OH D-Erythrulose CH2OH C O CH2OH Dihydroxyacetone Ketopentoses CH2OH C O H C OH H C OH CH2OH CH2OH C O HO C H H C OH CH2OH D-Ribulose D-Xylulose Ketohexoses CH2OH C O HO C H H C OH H C OH CH2OH CH2OH C O H C OH HO C H H C OH CH2OH D-Fructose D-Sorbose
- 6. Configuration and Conformation Alcohols can react with aldehydes or ketones to form hemiacetals or hemiketals. O H + C R OH R' C H O OH R' R hemiacetal O R'' + C R OH R' C R'' O OH R' R hemiketal The hydroxy group and the aldehyde (or ketone) of monosaccharides can react intramolecularly to form cyclic hemiacetals (or hemiketals).
- 7. Haworth Projections H CHO C OH HO C H H C OH H C OH CH2OH D-Glucose CH2OH H O OH H OH H H OH H OH H OH H H CH2OH H OH OH H OH O = CH2OH OH HOH2C H O H HO OH H CH2OH C O HO C H H C OH H C OH CH2OH D-Fructose CH2OH O HOH2C H OH H HO OH H = -D-Glucopyranose -D-Fructofuranose
- 8. Cyclic Sugars have two Anomeric Forms H CHO C OH HO C H H C OH H C OH CH2OH D-Glucose CH2OH H O OH OH H H H OH H OH -D-Glucopyranose CH2OH H O OH H OH H H OH H OH -D-Glucopyranose
- 9. Monosaccharides are conformationally variable Two chair conformations of -D-glucopyranose: HO O H H HO H H OH H OH OH OH O H OH H OH H OH H OH H Two chair conformations of -D-glucopyranose: HO O H H HO H H OH HO H OH H O OH OH H OH H OH H OH H
- 10. Monosaccharide Derivatives H CHO C OH HO C H H C OH H C OH COOH D-Glucuronic acid Oxidations: H COOH C OH HO C H H C OH H C OH CH2OH D-Gluconic acid Reductions: H CH2OH C OH H C OH H C OH CH2OH H CH2OH C OH HO C H H C OH CH2OH D-Ribitol D-Xylitol H Amino Sugars: CH2OH C OH CH2OH D-Glycerol H CH2OH HO CH2OH H OH OH H HO OH H H H OH H OH myo-Inositol H O HO H OH H H OH H NH2 -D-Glucosamine HO O H H OH H H NH2 -D-Galactosamine
- 11. Glycosides The anomeric carbon can condense with alcohols CH2OH H O HO H OH H H OH H OH -D-Glucose + CH3OH ( R-OH ) CH2OH H O HO H + + H2O OCH3 H H OH H OH Methyl--D-Glucoside CH2OH H O HO OCH3 H H H OH H OH Methyl--D-Glucoside ( O-R ) ( O-R ) to form -glycosides and -glycosides.
- 12. Polysaccharides (or Glycans) • Homopolysaccharides - consist of one type of monosaccharide • Heteropolysaccharides - consist of more than one type of monosaccharide • Polysaccharides form branched as well as linear polymers. • Complete description of an oligosaccharide includes: - identity of the monosaccharides - anomeric forms - linkages of all its component monosaccharides
- 13. Disaccharides - simplest polysaccharides Lactose occurs naturally in milk disaccharide of galactose and glucose 6 6 1 CH2OH H O OH 1 H H H OH H OH -D-Glucose CH2OH HO O H H OH H H H OH -D-Galactose O Systematic Name: O--D-galactopyranosyl-(14)-D-glucopyranose (14) designates that the glycosidic bond links C1 of galactose with C4 of glucose Lactose has a free anomeric carbon on the glucose end. This is an example of a reducing sugar, and it can readily reduce mild oxidizing agents.
- 14. Sucrose Sucrose most abundant dissacharide common table sugar major form of carbohydrate transported in plants 6 CH2OH H O H H H OH HOH2C H O H HO 1 2 O HO CH2OH H OH -D-Glucose OH H -D-Fructose Systematic Name: O--D-glucopyranosyl-(12)--D-fructofuranoside The anomeric carbon of glucose and the anomeric carbon of fructose participates in the glycosidic bond. 6 5
- 15. Structural Polysaccharide - Cellulose • Plants have rigid cell walls to withstand osmotic pressure differences and for load-bearing functions. • Cellulose is the primary structural component of plant cell walls. • Cellulose is a linear polymer of up to 15,000 D-glucose residues linked by (14) glycosidic bonds. CH2OH H O [ H O H ] H OH H OH -D-Glucose O CH2OH H O H H H OH H OH -D-Glucose n
- 16. Storage Polysaccharide - Starch • Starch is a mixture of glycans that plants synthesize as their principal food reserve. • Composed primarily of -amylose and -amylopectin. • The -amylose is a linear polymer of glucose residues linked by (14) bonds. n CH2OH H O H H OH H OH -D-Glucose CH2OH H O H H OH H OH H [ ] -D-Glucose O H O -amylose Fig. 8-10 Saliva contains amylase which hydrolyzes the (14) bonds of starch.
- 17. Amylopectin and Glycogen - branched polysaccharide • Amylopectin in plants and Glycogen in animals consist mainly of (14)-linked glucose residues but is a branched molecule with (16) branch points. (16) branch point (16) branch point n Amylopectin in plants O H CH2OH H OH H OH H ] O H O H CH2 H OH H OH H [ O H O O . . . . . . . . . . . . [ (16) branch points every 24 to 30 glucose residues n Glycogen in animals O H CH2OH H OH H OH H ] O H O H CH2 H OH H OH H [ O H O O . . . . . . . . . . . . [ (16) branch points every 8 to 12 glucose residues
- 18. Glycoproteins • Many proteins are actually glycoproteins with carbohydrates (varing from <1% to >90%). • Polypeptide chain is synthesized under genetic control. • The carbohydrate is added by posttranslational modification. Proteoglycans = complex mixture consisting of proteins and glycosaminoglycans. a proteoglycan Fig. 8-13
Notas do Editor
- 1
- 12
- 18
