9/25 What is the trend for electronegativity?
•
0 gostou•1,340 visualizações
Denunciar
Compartilhar
Denunciar
Compartilhar
Baixar para ler offline
Recomendados
Recomendados
Mais conteúdo relacionado
Mais procurados
Mais procurados (20)
Destaque
Destaque (8)
Semelhante a 9/25 What is the trend for electronegativity?
Semelhante a 9/25 What is the trend for electronegativity? (20)
10/13 Review: What is electronegativity and ionization energy?

10/13 Review: What is electronegativity and ionization energy?
B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...

B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...
Mais de mrheffner
Mais de mrheffner (20)
Último
Último (20)
AWS Community Day CPH - Three problems of Terraform

AWS Community Day CPH - Three problems of Terraform
ProductAnonymous-April2024-WinProductDiscovery-MelissaKlemke

ProductAnonymous-April2024-WinProductDiscovery-MelissaKlemke
Scaling API-first – The story of a global engineering organization

Scaling API-first – The story of a global engineering organization
Boost Fertility New Invention Ups Success Rates.pdf

Boost Fertility New Invention Ups Success Rates.pdf
Cloud Frontiers: A Deep Dive into Serverless Spatial Data and FME

Cloud Frontiers: A Deep Dive into Serverless Spatial Data and FME
Strategize a Smooth Tenant-to-tenant Migration and Copilot Takeoff

Strategize a Smooth Tenant-to-tenant Migration and Copilot Takeoff
Strategies for Landing an Oracle DBA Job as a Fresher

Strategies for Landing an Oracle DBA Job as a Fresher
Bajaj Allianz Life Insurance Company - Insurer Innovation Award 2024

Bajaj Allianz Life Insurance Company - Insurer Innovation Award 2024
Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood

Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood
TrustArc Webinar - Stay Ahead of US State Data Privacy Law Developments

TrustArc Webinar - Stay Ahead of US State Data Privacy Law Developments
From Event to Action: Accelerate Your Decision Making with Real-Time Automation

From Event to Action: Accelerate Your Decision Making with Real-Time Automation
Apidays New York 2024 - The value of a flexible API Management solution for O...

Apidays New York 2024 - The value of a flexible API Management solution for O...
Automating Google Workspace (GWS) & more with Apps Script

Automating Google Workspace (GWS) & more with Apps Script
Bajaj Allianz Life Insurance Company - Insurer Innovation Award 2024

Bajaj Allianz Life Insurance Company - Insurer Innovation Award 2024
Tata AIG General Insurance Company - Insurer Innovation Award 2024

Tata AIG General Insurance Company - Insurer Innovation Award 2024
Workshop - Best of Both Worlds_ Combine KG and Vector search for enhanced R...

Workshop - Best of Both Worlds_ Combine KG and Vector search for enhanced R...
9/25 What is the trend for electronegativity?
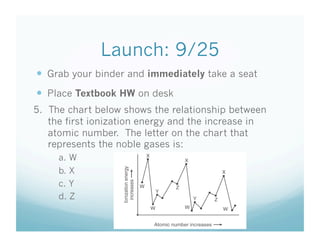
- 1. Launch: 9/25 Grab your binder and immediately take a seat Place Textbook HW on desk 5. The chart below shows the relationship between the first ionization energy and the increase in atomic number. The letter on the chart that represents the noble gases is: a. W b. X c. Y d. Z
- 2. What is the trend for electronegativity? Mr. Heffner 9/25/09
- 3. Review: Atomic Radius Decreases Increases
- 4. IV. Ionization Energy Increases Decreases
- 5. V. Electronegativity A. What is electronegativity? 1. The ability of an atom to attract electrons toward itself in a chemical bond H O H
- 6. V. Electronegativity B. What is the trend for electronegativity? 1. Electronegativity increases across a period (LR) i. As the number of protons increases, the nucleus gets stronger and pulls all nearby electrons towards it
- 7. V. Electronegativity B. What is the trend for electronegativity? 2. Electronegativity decreases down a group (TopBottom) i. As the number of electrons increases, more rings are added ii. The inner rings shield other electrons from the attractive forces of the nucleus
- 8. V. Electronegativity Increases Decreases
- 9. Practice Questions See Periodic Trends Worksheet
- 10. Homework Study for Monday’s Unit #2 Exam! Look over Quiz #3 Look over Exit Slips lpschem.wordpress.com
- 11. Exit Slip 1. Electronegativity is the a. energy it takes to remove an electron from an atom. b. number of protons and neutrons in an atom. c. ability of an atom to attract electrons toward itself in a chemical bond d. distance from the nucleus to the valence ring.
- 12. Exit Slip 2. In general, how does electronegativity vary throughout the periodic table? a. it decreases across a period from left to right, and decreases down a group from top to bottom b. it increases across a period from left to right, and increases down a group from top to bottom c. it increases across a period from left to right, and decreases down a group from top to bottom d. It decreases across a period from left to right, and increases down a group from top to bottom
- 13. Exit Slip 3. Why does electronegativity decrease down a group? a. Because atomic radius decreases b. Because the nucleus is shielded by the inner rings c. Because the outer electrons are more strongly attracted by the nucleus d. Because the trend for electronegativity increases down a group
- 14. Exit Slip 4. Which of the following atoms is more electronegative than S? a. oxygen (O) b. phosphorus (P) c. selenium (Se) d. magnesium (Mg)
- 15. Exit Slip 5. Which of the following atoms is the most electronegative? a. sodium (Na) b. phosphorus (P) c. selenium (Se) d. magnesium (Mg)