Case record...Intraventricular ependymoma
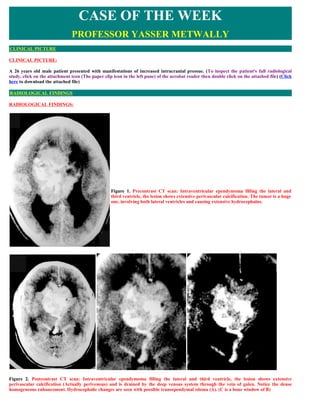
- 1. CASE OF THE WEEK PROFESSOR YASSER METWALLY CLINICAL PICTURE CLINICAL PICTURE: A 26 years old male patient presented with manifestations of increased intracranial pressue. (To inspect the patient's full radiological study, click on the attachment icon (The paper clip icon in the left pane) of the acrobat reader then double click on the attached file) (Click here to download the attached file) RADIOLOGICAL FINDINGS RADIOLOGICAL FINDINGS: Figure 1. Precontrast CT scan: Intraventricular ependymoma filling the lateral and third ventricle, the lesion shows extensive perivascular calcification. The tumor is a huge one, involving both lateral ventricles and causing extensive hydrocephalus. Figure 2. Postcontrast CT scan: Intraventricular ependymoma filling the lateral and third ventricle, the lesion shows extensive perivascular calcification (Actually perivenous) and is drained by the deep venous system through the vein of galen. Notice the dense homogeneous enhancement. Hydrocephalic changes are seen with possible transependymal edema (A). (C is a bone window of B)
- 2. Figure 3. The tumor has extended to the third ventricle, ? the interhemispheric fissure, and Probably the suprasellar and the quadrigeminal cistern. CSF seedling is probably the cause of dissemination through the basal cisterns and the bihemispheric fissures. Figure 4. Angiography shows the extensive tumour hypervascularity that is mainly drained by the deep venous system through the vein of galen. The patient's tumor was debulked surgically and histopathological examination revealed an ependymoma of the cellular type with Perivascular pseudorosettes. The patient was given a course of postoperative radiotherapy (Fig. 5). The patient died 7 month following the operation. Comment Ependymomas constitute 2% to 6% of all gliomas. Intracranial ependymomas are most common in the first and second decades but are seen in adults. Of intracranial ependymomas, 30% to 40% occur in the supratentorial compartment. The majority of these supratentorial ependymomas are parenchymal in location rather than intraventricular, with the reported frequency of a parenchymal origin ranging from about 56% to 85%. This is because subependymal neural glia may extend into adjacent white matter as a streak or a band, leading to ependymal cell rests remote from the ventricular lining. This tends to occur particularly where the ventricles are sharply angled. In the supratentorial compartment, these tumors have a predilection for the frontal and parietal lobes and are usually large at the time of diagnosis (94% of tumors are larger than 4 cm ). Characteristically they are well defined and homogeneous in appearance, but larger tumors may undergo cystic degeneration. Hemorrhage is not a characteristic feature. Microscopically there may be a variable appearance both between different tumors and in different parts of the same tumor. The pathognomonic histologic feature is the appearance of ependymal rosettes, but these are not frequently seen.
- 3. Figure 5. Histopathological picture of ependymomas with the characteristic rosettes (The patient's histopathology) Rarely malignant or anaplastic ependymomas may occur with rapid growth and recurrence after surgery, although still with a relatively favorable clinical course compared with other gliomas. Ependymoblastomas are considered a rare embryonal form of ependymal neoplasm occurring in the supratentorial compartment in children. These tumors are usually large, frequently invade the leptomeninges, and disseminate through the subarachnoid space. They have a poor prognosis. Subependymomas are another variant of ependymoma with a significant astroglial component to the tumor, although microscopically still having characteristic ependymal cellular features. These lesions tend to be intraventricular and multiple, slow growing, and benign in behavior. They most frequently arise in the fourth ventricle but occur at other sites, including the lateral ventricles. Large subependymomas may be pedunculated and contain focal areas of cystic degeneration, hemorrhage, and calcification. These tumors may remain small and asymptomatic, being discovered incidentally on imaging studies or at postmortem. Larger lesions may produce symptoms secondary to ventricular obstruction and hydrocephalus. Supratentorial ependymomas are usually large frontoparietal masses. Although dense punctate calcifications are seen in about 50% of CT scans, overall the tumor is usually hypodense or isodense relative to adjacent brain; intratumoral hemorrhage is not a feature. Hydrocephalus is frequently seen in infratentorial tumors but is rare in supratentorial lesions. Perifocal edema and cystic change within the tumor are each seen in about half the cases. The tumor margins may vary from poorly to well defined. There is moderate to marked enhancement after contrast administration; the pattern is variable, ranging from homogeneous to inhomogeneous or ring enhancing. MR imaging scanning, with sagittal and coronal images, demonstrates the route of spread of intraventricular ependymomas and subependymomas well. On Tl-weighted images, the solid components of supratentorial ependymomas are hypointense to isointense and are hyperintense on T2-weighted images; areas of cystic degeneration usually have similar signal intensities to cerebrospinal fluid. There may, however, be heterogeneity of signal intensity in solid tumors owing to the presence of blood products (although hemorrhage occurs infrequently), necrosis, or calcification, which may not be recognized on spin echo images but is identified on gradient echo pulse sequences. Edema appears as regions of increased signal intensity in adjacent white matter on T2-weighted images. Enhancement is usually moderate to intense and may vary in its pattern from patchy to homogeneous. On angiography a number of cases with intraventricular ependymomas have been seen presenting a relatively homogeneous tumor cloud which tended to persist, although the masses are not sharply circumscribed or lobulated, as occurs with meningiomas. Early draining veins could be seen in the area of the tumor in some cases. A few mixed intraventricular ependymomas have been observed presenting increased vascularity and rapid venous drainage by way of branches of the thalamostriate vein. These can usually be identified as intraventricular because of their location close to the midline in the frontal projection, and in the region of the ventricles in the lateral projection; at times they have the shape of a lateral ventricle. Some of these intraventricular ependymomas are so vascular that the possibility of a vascular malformation is suggested . Differentiation from a vascular malformation is possible because even though the draining veins fill early and are numerous, they do not empty as fast with a glioma as with an arteriovenous malformation. DIAGNOSIS: DIAGNOSIS: INTRAVENTRICULAR SUPRATENTORIAL EPENDYMOMA DISCUSSION DISCUSSION: Intraventricular Ependymoma Ependymomas are common neoplasms that arise from differentiated ependymal cells that line the cerebral ventricles and the central canal of the spinal cord (1). They constitute 3%–9% of all neuroepithelial neoplasms, 6%–12% of all pediatric brain tumors, and almost one- third of all brain tumors in patients younger than 3 years (1). Ependymomas may manifest at any age, with a documented age range of 1 month to 81 years (1). There is no gender predilection (1). Most posterior fossa ependymomas arise in children, with a mean age of about 6 years (1). The mean age at presentation is higher for patients who have a supratentorial ependymoma (18–24 years) (2). Of those ependymomas that occur intraventricularly, 58% originate in the fourth ventricle, whereas the remaining 42% are located in the lateral and third ventricles (3). Supratentorial ependymomas are more commonly extraventricular, especially in children (1,2). It is speculated that ependymomas may arise from embryonic rests of ependymal tissue trapped within the developing cerebral hemispheres (1). Bizarre sites reported for ependymomas include the ovaries, soft tissues, mediastinum, and sacrococcygeal region (4). As with other intraventricular masses, clinical signs and symptoms are largely secondary to the effects of increased intracranial pressure
- 4. and hydrocephalus (1). Because the fourth ventricle is a predominant site for these tumors, some patients may present with cerebellar ataxia and paresis (1,5). Patients with supratentorial ependymomas tend to present with focal neurologic deficits and seizures (1). In general, children with ependymomas have a less favorable prognosis than adults, in part from the increased prevalence of a fourth ventricle location and the predilection of this group for more anaplastic forms of the disease (1). The 5-year progression-free rate for children overall is about 50% (6), with children younger than 2 years having an especially poor prognosis (7). The 5-year and 10-year survival rates for adults are 57.1% and 45%, respectively (1). The treatment of choice is gross total resection, and the degree of resection directly correlates with a better prognosis (8). Patients with supratentorial ependymomas have a better survival rate than those patients with posterior fossa ependymomas (9). For all types of ependymomas, recurrence is common (2,10). Postoperative radiation therapy is advocated for partially resected ependymomas (10). Intraventricular ependymomas are well-circumscribed, grayish-red masses that usually fill the ventricular lumen and occasionally may extend into the adjacent brain parenchyma (Fig 1) (1). When they arise in the fourth ventricle, these soft pliable tumors originate from the floor or roof of the ventricle and frequently extend through the foramen of Luschka into the cerebellopontine angle and even the foramen magnum (Fig 2) (1). Figure 1. Ependymoma. Photograph of an autopsy specimen sectioned through the level of the fourth ventricle shows a soft, friable heterogeneous mass (arrows) within the fourth ventricle. Scattered areas of hemorrhage are noted. Figure 2. Ependymoma. Photograph of a brain at autopsy shows extensive cerebellopontine angle extension (arrows) from a fourth ventricular ependymoma. Numerous areas of hemorrhage give the mass a heterogeneous appearance. At histologic analysis, ependymomas are moderately cellular tumors characterized by rare mitotic figures; perivascular pseudorosettes; and, less commonly, ependymal rosettes (Fig 3) (1). Accordingly, they are considered World Health Organization (WHO) grade II lesions (1). Several variant forms are also noted, based on histopathologic features, and include cellular ependymoma, papillary ependymoma, clear cell ependymoma, tanycytic ependymoma, and anaplastic ependymoma (WHO grade III) (1).
- 5. Figure 3. Ependymoma. Photomicrograph (original magnification, x100; hematoxylin-eosin stain) of an ependymoma shows a moderately cellular matrix composed of glial cells with perivascular rosettes (arrowheads) and no mitotic figures. On nonenhanced computed tomographic (CT) images, intraventricular ependymomas are usually isoattenuated, partially calcified masses (11). The soft-tissue component shows intense enhancement on contrast material–enhanced images (11). The soft-tissue portion of the tumor is usually hypoattenuated to isoattenuated on nonenhanced CT images (12). Occasionally, intratumoral hemorrhage may produce a blood-fluid level (2). Calcification, ranging from small punctate foci to large masses, is common (40%–80% of cases) (Fig 4) (2,11). Contrast enhancement is variable but usually intense within the soft-tissue portions, although it spares the cystlike regions (2,11). In contrast to most posterior fossa ependymomas, supratentorial ependymomas are usually located in the cerebral parenchyma and frequently have a cystic appearance on cross-sectional images (2,11,13). Figure 4. Ependymoma in a 16-month-old child. (a) Axial CT image shows a fourth ventricular mass that is slightly hyperattenuated compared with the surrounding cerebellum. Focal calcification (arrow) is noted. (b) Axial T1-weighted magnetic resonance (MR) image shows the mildly heterogeneous mass, which is slightly hypointense compared with the cerebellum. (c) On an axial T2-weighted image, the mass is hyperintense compared with the cerebellum, with no surrounding vasogenic edema. (d) On a contrast-enhanced axial T1-weighted image, the mass shows intense heterogeneous enhancement On MR images, intraventricular ependymomas typically show isointensity compared with gray matter with short repetition time (TR) pulse sequences and hyperintensity compared with gray matter with long TR pulse sequences. A heterogeneous appearance is typical, reflecting the calcification, hemorrhage, and cystic changes that are often present (Figs 4–6). As seen on CT images, there is variable contrast enhancement (2). Supratentorial ependymomas typically show nonspecific hypointensity to isointensity with short TR pulse sequences and hyperintensity with long TR pulse sequences (2).
- 6. Figure 5. Malignant ependymoma in a 58-year-old woman with a history of ductal carcinoma and renal cell carcinoma. (a) Axial CT image shows a heterogeneously hypoattenuated mass (arrows) adjacent to the right frontal horn of the lateral ventricle. (b) Axial T1-weighted MR image shows the heterogeneous mass, with cystlike areas mixed with more hyperintense regions. (c) On an axial T2-weighted MR image, the mass is predominantly hyperintense with focal regions of more hypointense signal. (d) On a contrast-enhanced axial T1-weighted MR image, the mass shows intense but heterogeneous enhancement. At surgery, the mass involved both the lateral ventricle and the right frontal lobe. Histologic examination revealed ependymoma. Figure 6. Ependymoma in a 29-year-old adult. (a) Sagittal T1-weighted MR image shows an isointense fourth ventricular mass (arrows) with inferior extension through the foramen magnum. (b) On an axial T2-weighted MR image, the mass is mildly hyperintense compared with the cerebellum and extends through the right foramen of Luschka into the cerebellopontine angle. (c) On a contrast-enhanced axial T1-weighted MR image, the mass shows intense but heterogeneous enhancement. MR imaging is considered the modality of choice to evaluate these lesions, although CT is superior in the detection of calcification (2). Postoperative imaging is considered crucial in documenting the presence of postoperative residual disease, which has a substantial negative impact on survival rates in patients with this tumor (14). Intraventricular Subependymoma In 1945, Scheinker (15) described seven cases of brain tumors arising from the subependymal glial layer surrounding the cerebral ventricles. These tumors were characterized by expansive growth and lacked an infiltrative pattern at histologic examination. Since that time, more than 100 similar cases have been reported in the literature. With the exception of a very small number of cases that occurred in the brain parenchyma, cerebellopontine angle, and spinal cord, the overwhelming majority of these tumors have occurred within the fourth and lateral ventricles (2,5,15–25). The true incidence of subependymomas is difficult to ascertain because many of the cases occurred in asymptomatic patients and were detected only incidentally at autopsy. In a review of 1,000 serial necropsies in asymptomatic patients, Matsumura at al (17) reported the prevalence as 0.4%, compared with a prevalence of 0.7% in 1,000 consecutive craniotomies
- 7. performed in symptomatic patients. Despite four case reports of subependymomas occurring in siblings, including one set of identical twins, no genetic susceptibility for the tumor has been proved (19,21,26). Most subependymomas are smaller than 2 cm in diameter (26). However, symptomatic subependymomas are usually larger, averaging about 3–5 cm in greatest dimension (5,22). Symptoms most commonly depend on the location and size of the tumor, with intratumoral hemorrhage being another possible influence (5). The clinical presentation is nonspecific. Most symptomatic patients (80%) present with symptoms related to hydrocephalus as a consequence of ventricular obstruction (16,26). Less commonly, focal neurologic deficits (27% of cases), seizures (9%), and subarachnoid hemorrhage (4.5%) have been reported (16). Males are more commonly affected, and most reported cases (82%) have occurred in patients older than 15 years (16,26). At least half of the reported cases have occurred in the fourth ventricle, with most of the remainder arising in the lateral ventricle (2,5,15–26). In rare cases, subependymomas have been recorded arising from the septum pellucidum, the third ventricle, and in the cervical or cervicothoracic spinal cord (16, 23). Gross total surgical resection is the goal of therapy (16). Even if only partial resection is achieved, postoperative radiation therapy or chemotherapy is usually not indicated. Recurrence after surgical resection is rare (2,22). Subependymomas have a white to grayish color and are well circumscribed with a firm texture (16). The tumors grow in a slow deliberate fashion, are usually avascular, and are attached to the ventricular wall by a narrow pedicle (16). Although the exact histogenesis is still uncertain, they most likely arise from subependymal glial cells (26). Other possible sites include astrocytes from the subependymal plate, ependymal cells, and a mixture of ependymal and astrocytic cells (26). A dense fibrillary matrix interrupted by numerous small cysts and nests of isomorphic nuclei that resemble subependymal glia is typically seen at histologic examination (Fig 7) (26). Mitotic activity is usually low or absent; thus, subependymomas correspond histologically to WHO grade I (26). Although most tumors are pure subependymomas, about 10% may manifest as an admixture with an ependymoma (5,16,26). In addition, other reported combinations include those with melanin, rhabdomyosarcoma, and sarcomatous transformation of vascular stromal elements (26). The prognosis of a patient with an intraventricular subependymoma is good, with gross surgical resection being curative (26). A good clinical outcome is less certain when the tumor is mixed with an ependymoma (5). Figure 7. Subependymoma. Photomicrograph (original magnification, x40; hematoxylin-eosin stain) of a subependymoma shows scattered clusters of nuclei (arrowheads) separated by large, acellular regions of glial processes The typical CT appearance of a subependymoma is a well-circumscribed, lobulated intraventricular mass that is predominantly isoattenuated to slightly hypoattenuated compared with the brain parenchyma (Fig 8) (16,22). Hydrocephalus is present in 85% of cases (16). When hemorrhage is present, the mass may show hyperattenuation compared with the brain parenchyma (17,18). Most (84%) show at least some enhancement, more likely focal in nature, on contrast-enhanced images (16). Calcification (31.8% of cases) and cystic degeneration (18%) are common (16,22). Dense calcification is not common (2,16,23). Occasionally, subependymomas may produce peritumoral edema on cross-sectional images (2,16). Although most are avascular, some may have a blush on angiographic studies from discrete tumor vascularization (17).
- 8. Figure 8. Subependymoma in a 53-year-old man. (a) Axial CT image shows a right frontal horn mass that is predominantly isoattenuated compared with the brain parenchyma. Curvilinear calcification (arrow) is seen. (b) Axial T1-weighted MR image shows isointensity within the mass, compared with the white matter. (c) Axial T2- weighted MR image shows heterogeneous hyperintensity within the mass and no evidence of periventricular edema. (d) Contrast-enhanced axial T1-weighted MR image shows scattered heterogeneous enhancement within the mass. In attempting to differentiate subependymomas from ependymomas with imaging studies alone, Lobato et al (16) noted that subependymomas tend to be intraventricular, whereas ependymomas tend be paraventricular. They also reported that hyperattenuation compared with the brain parenchyma, enhancement, calcification, and cyst formation were also more commonly seen in ependymomas than in subependymomas (16). However, none of these features are sufficiently pronounced to be pathognomonic for either lesion. The distinctions between these tumors are even less apparent for those that arise in the fourth ventricle (16). On MR images, subependymomas are generally hypointense compared with white matter with short TR pulse sequences and hyperintense compared with white matter with long TR pulse sequences (Figs 8 –10) (22–24). Heterogeneity is typical, with cystlike areas interspersed within the mass (22,24). When hemorrhage is present, characteristic signal intensity representative of hemoglobin by-products is noted (23). Enhancement is quite variable on contrast-enhanced images (22,23). They may not enhance, enhance minimally, or show intense enhancement after the intravenous administration of a contrast agent (24). Even when intense enhancement is seen, it is usually heterogeneous (2,24). Extension of a subependymoma beyond the ventricular margins is rare (23,27). These features may be helpful in distinguishing subependymomas from ependymomas, since the latter frequently have intense enhancement and extraventricular extension (24). Figure 9. Subependymoma in a 70-year-old woman in whom breast carcinoma had been diagnosed 7 years before. A metastatic lesion to the chest wall had been found 4 months before she experienced a sudden onset of weakness and slurred speech that prompted neuroimaging. (a) Sagittal T1-weighted MR image shows a mass that is isointense compared with the white matter and that extends inferiorly. A sellar mass of unknown pathologic characteristics erodes the floor of the sella turcica. (b) Axial T2-weighted MR image shows a fourth ventricular mass (arrows) that is slightly hyperintense compared with the white matter. A metastasis was suspected. (c) Intraoperative photograph shows the well-circumscribed, firm, glistening mass (arrows) in the inferior portion of the fourth ventricle. Findings from histologic examination confirmed subependymoma, not metastatic disease
- 9. Figure 10. Subependymoma in a 48-year-old man with a history of nausea for several months. Results from a prior abdominal CT study and endoscopy were negative. After developing new headaches, he presented again for evaluation. (a) Sagittal T1-weighted MR image shows a soft-tissue mass (arrows) in the inferior portion of the fourth ventricle with extension through the foramen magnum. Note dilatation of the remaining portions of the ventricular system as a result of the mass. (b) On an axial T2-weighted MR image, the mass appears heterogeneous, with cystic and soft-tissue components. Note absence of vasogenic edema. (c) Contrast-enhanced sagittal T1- weighted MR image shows intense heterogeneous enhancement of the mass. SUMMARY SUMMARY Epidemiology of intracellular ependymoma Ependymomas are neoplasms derived from the ependymal layer lining the ventricular system and can occur intracranially and in the spine. Intracranial ependymomas account for 2% to 8% of all primary CNS neoplasms [26], with more than half presenting in the first two decades of life. In a series of 467 pediatric intracranial neoplasms reviewed by Farwell et al [27], ependymomas made up 9% of all intracranial tumors, making it the third most common pediatric intracranial tumor. Within the pediatric population, ependymomas favor young patients, with more than 50% occurring within the first 3 years of life. No consistent gender predilection has been identified. Intracranial ependymomas can be divided by location into those appearing infratentorially and those appearing supratentorially. Infratentorial ependymomas make up approximately two thirds of all cases [28], comprise most pediatric cases, and most frequently occur in the fourth ventricle [40]. Supratentorial ependymomas occur more frequently in older children and adults. In addition to the lateral ventricles, approximately 50% of supratentorial ependymomas involve the parenchyma [29]. Macroscopic and microscopic features of intracellular ependymoma Ependymomas are often sharply demarcated, fleshy, hemorrhagic, soft, and sometimes rubbery masses. Rare examples are heavily calcified, giving the tumor a gritty texture. Intraventricular examples of ependymomas are often lobulated and display a discrete interface with surrounding brain. Some tumors may exhibit a delicate overlying ependymal layer that gives them a shiny texture. Ependymomas are glial neoplasms composed of a monomorphous proliferation of neoplastic cells with typical “perivascular pseudorosettes” (see Fig. 1C, D). Some ependymomas are predominantly glial in appearance and may not have distinct perivascular pseudorosettes, whereas others may be predominantly epithelial. The latter may present as a tumor with oval to round nuclei, discrete cytoplasmic borders, frank papillary structures, and well-formed fibrovascular cores. Other tumors may show “true ependymal rosettes” distinguished by their well-defined lumina and cells forming pseudoglandular structures. Most ependymomas show a substantial number of nuclear grooves that can be identified in intraoperative smears and help with the rapid interpretation of frozen sections [30]. This feature, however, needs to be interpreted in the context of other histologic findings, because many other tumors, such as meningiomas and other gliomas, can exhibit nuclear grooves. The tumor nuclei are uniform, round to oval, and often feature a distinct nucleolus. Clear cell change in ependymoma is a rare but significant finding [31]. Intraventricular ependymomas may exhibit focal or predominant clear cell change. When clear cell change is predominant, the hematoxylin-eosin appearance of an oligodendroglioma is recapitulated. It is likely that many tumors previously reported as intraventricular oligodendroglioma are examples of clear cell ependymoma [21]. Clear cell ependymomas are usually higher grade and exhibit increased mitotic activity and vascular proliferation. The so-called “tanycytic ependymoma” is remarkably similar to a pilocytic astrocytoma. This highly fibrillary tumor has moderate cell density, spindled cells, and a fascicular architecture. It has also been described as a “piloid tumor with ependymal nuclei” [32]. The tanycytic ependymoma often lacks nuclear pleomorphism or aggressive features, such as mitoses or vascular proliferation. Perivascular pseudorosettes are rudimentary and sometimes absent.
- 10. Ependymomas are commonly calcified and rarely exhibit cartilaginous and osseous metaplasia. Rare ependymomas contain cytoplasmic eosinophilic granules, clear vacuoles, lipid, or melanin [33,34]. The current WHO classification defines grade II ependymomas as tumors with mild cellular pleomorphism, pseudorosettes, or true ependymal rosettes. The tumors can have occasional mitotic figures and necrosis without pseudopalisading. Occasional foci of hypercellularity and increased mitoses are allowed. “Anaplastic,” “high-grade,” or grade III ependymomas have moderate to high cellularity, increased mitotic figures, and vascular proliferation. Necrosis is often present, either in the form of geographic necrosis or, rarely, in the pseudopalisading form. Perivascular pseudorosettes or occasional true ependymal rosettes can be found in most high-grade ependymomas. There is controversy around whether focal “atypia” or “anaplasia” should elevate a lesion to grade III “anaplastic ependymoma.” Some require atypia and anaplasia to predominate in the tumor tissue, whereas others report a less favorable prognosis even for tumors with focal anaplastic features. Immunohistochemical features of intracellular ependymoma Ependymomas are variably positive for GFAP, which highlights the fibrillary processes around vessels. Tumors are diffusely positive for vimentin and stain less avidly with S-100 protein and neurospecific enolase (NSE). Positive staining for epithelial markers, such as EMA and cytokeratins, has been reported in most posterior fossa and spinal cord ependymomas [35]. Rare tumor cells, true rosettes, and occasional papillary structures are EMA-positive. Studies suggest that high Ki-67/MIB-1 and p53 protein positivity might be reliable indicators of high-grade ependymomas [36]. Even though there seems to be a positive correlation between high-grade features and the Ki-67/MIB-1 index [37], none of the immunohistochemical variables significantly correlate with tumor grade. Conversely, Ki-67/MIB-1 and p53 were reported to correlate with patient survival [39]. Currently, there is no clear evidence for the utility of these markers in determination of tumor grade or behavior. Ultrastructural features of intracellular ependymoma The acellular zones around pseudorosettes are composed of large numbers of closely packed, filament-rich, cytoplasmic processes. Microlumina are often present, even though they may not be observed by light microscopy [40]. These microlumina contain slender curving microvilli and a variable number of cilia. Bordering cells are connected by unusually long tight junctions. This triad (cilia, intracytoplasmic intermediate filaments, and cell junctions) makes up the typical ultrastructural components. The epithelioid cells found in ependymomas and true rosettes are characterized by intracellular lumina, cilia, and microvilli. Clear cell ependymomas reveal densely packed polyhedral cells with clear cytoplasm and well-developed intercellular junctions. Abundant hyaloplasmic lipid vacuoles can also contribute to the clear appearance of the tumor cells [41]. Molecular and genetic features of intracellular ependymoma There is a body of evidence suggesting the presence of a tumor suppressor gene on the long arm of chromosome 22 that plays a role in the pathogenesis of ependymomas [42]. In one study, the most frequent copy number abnormality in ependymomas was 22q loss, followed by gain of chromosome 9 and occasional loss of 6q, 3p, 10q, and 15q [41]. A heterozygous mutation in the MEN1 gene has also been reported in ependymomas [42]. Pathologic differential diagnosis of intracellular ependymoma Formulation of the differential diagnosis for ependymoma is dependent on the location of the lesion. In the posterior fossa, medulloblastoma needs to be considered first in the differential diagnosis, although its architecture is more reminiscent of a small blue round cell tumor than that of a glioma. Pilocytic astrocytoma of the cerebellum or brain stem is a second possibility but can be easily excluded when classic features of pilocytic astrocytomas, such as Rosenthal fibers, eosinophilic granular bodies, and a fairly paucicellular appearance, are present. Infiltrating astrocytomas or the so-called “brain stem gliomas” may have an exophytic quality and may resemble ependymoma. They are easily distinguished by their invasive quality, lack of epithelial features or pseudorosettes, and marked nuclear pleomorphism. Supratentorial intraventricular ependymomas need to be distinguished from subependymomas. Such distinction is often subjective and may not always translate into a significant change in clinical outcome. Nevertheless, based on the overall clinical behavior of ependymomas and the likelihood of supratentorial examples being higher grade, one is compelled to make the distinction. The distinction is usually not difficult, and the differential diagnosis is confounded by limited tissue sample size. A second yet more important differential diagnosis is oligodendroglioma, which can easily be confused with clear cell ependymoma. Clear cell ependymomas are noninfiltrating, solid, and distinct from the surrounding brain. Purely intraventricular neoplasms are not likely to be oligodendrogliomas, but when a question exists, immunohistochemistry and electron microscopy readily settle the issue. Another diagnostic consideration is the central neurocytoma. The central neurocytoma is a highly cellular neoplasm that may show perivascular pseudorosettes. The cells appear more neurocytic, and the fibrillar areas resemble neuropil. The tumor strongly reacts with synaptophysin and only weakly (if at all) with GFAP. Electron microscopy can distinguish the two entities. Papillary ependymomas may resemble CPP. The overall immunohistochemical profile and ultrastructural features can be used to separate the two entities. Addendum A new version of this PDF file (with a new case) is uploaded in my web site every week (every Saturday and remains available till Friday.) To download the current version follow the link "http://pdf.yassermetwally.com/case.pdf". You can also download the current version from my web site at "http://yassermetwally.com".
- 11. To download the software version of the publication (crow.exe) follow the link: http://neurology.yassermetwally.com/crow.zip The case is also presented as a short case in PDF format, to download the short case follow the link: http://pdf.yassermetwally.com/short.pdf At the end of each year, all the publications are compiled on a single CD-ROM, please contact the author to know more details. Screen resolution is better set at 1024*768 pixel screen area for optimum display. Also to view a list of the previously published case records follow the following link (http://wordpress.com/tag/case-record/) or click on it if it appears as a link in your PDF reader To inspect the patient's full radiological study, click on the attachment icon (The paper clip icon in the left pane) of the acrobat reader then double click on the attached file. Click here to download the short case version of this case record in PDF format REFERENCES References 1. Wiestler O, Schiffer D, Coons S, Prayson R, Rosenblum M. Ependymoma. In: Kleihues P, Cavenee W, eds. Pathology and genetics of tumours of the central nervous system. Lyon, France: IARC, 2000; 72-76. 2. Furie D, Provenzale J. Supratentorial ependymomas and subependymomas: CT and MR appearance. J Comput Assist Tomogr 1995; 19:518-526. 3. Schiffer D, Chio A, Giordana M, et al. Histologic prognostic factors in ependymoma. Childs Nerv Syst 1991; 7:177-182. 4. Morantz R, Kepes J, Batnitzky S, Masterson B. Extraspinal ependymomas: report of three cases. J Neurosurg 1979; 51:383-391. 5. Scheithauer B. Symptomatic subependymoma: report of 21 cases with review of the literature. J Neurosurg 1978; 49:689-696. 6. Robertson P, Zeltzer P, Boyett J, et al. Survival and prognostic factors following radiation therapy and chemotherapy for ependymomas in children: a report of the Children’s Cancer Group. J Neurosurg 1998; 88:695-703. 7. Kudo H, Oi S, Tamaki N, Nishida Y, Matsumoto S. Ependymoma diagnosed in the first year of life in Japan in collaboration with the International Society for Pediatric Neurosurgery. Childs Nerv Syst 1990; 6:375-378. 8. Pollock I, Gerszten P, Martinez A, et al. Intracranial ependymomas of childhood: long-term outcome and prognostic factors. Neurosurgery 1995; 37:655-667. 9. Ernestus R, Schroder R, Stutzer H, Klug N. Prognostic relevance of localization and grading in intracranial ependymomas of childhood. Childs Nerv Syst 1996; 12:522-526. 10. Palma L, Celli P, Cantore G. Supratentorial ependymomas of the first two decades of life: long-term follow-up of 20 cases (including two subependymomas). Neurosurgery 1993; 32:169-175. 11. Swartz J, Zimmerman R, Bilaniuk L. Computed tomography of intracranial ependymomas. Radiology 1982; 143:97-101. 12. McConachie N, Worthington B, Cornford E, Balsitis M, Kerslake R, Jaspan T. Review article: computed tomography and magnetic resonance in the diagnosis of intraventricular cerebral masses. Br J Radiol 1994; 67:223-243. 13. Armington W, Osborn A, Cubberley D, et al. Supratentorial ependymoma: CT appearance. Radiology 1985; 157:367-372. 14. Healey E, Barnes P, Kupsky W, et al. The prognostic significance of postoperative residual tumor in ependymoma. Neurosurgery 1991; 28:666-672. 15. Scheinker I. Subependymoma: a newly recognized tumor of subependymal derivation. J Neurosurg 1945; 2:232-240. 16. Lobato R, Sarabia M, Castro S, et al. Symptomatic subependymoma: report of four new cases studied with computed tomography and review of the literature. Neurosurgery 1986; 19:594-598. 17. Matsumura A, Ahyai A, Hori A, Schaake T. Intracerebral subependymomas: clinical and neuropathological analyses with special reference to the possible existence of a less benign variant. Acta Neurochir Wien 1989; 96:15-25. 18. Lindboe C, Stolt-Nielsen A, Dale L. Hemorrhage in a highly vascularized subependymoma of the septum pellucidum: case report. Neurosurgery 1992; 31:741-745. 19. Cheng T, Coffey R, Gelber B, Scheithauer B. Simultaneous presentation of symptomatic subependymomas in siblings: case reports and review. Neurosurgery 1993; 33:145-150. 20. Kim D, Han M, Lee S, et al. MRI of intracranial subependymoma: report of a case. Neuroradiology 1993; 35:185-186. 21. Ryken T, Robinson R, Van GJ. Familial occurrence of subependymoma: report of two cases. J Neurosurg 1994; 80:1108-1111.
- 12. 22. Chiechi M, Smirniotopoulos J, Jones R. Intracranial subependymomas: CT and MR imaging features in 24 cases. AJR Am J Roentgenol 1995; 165:1245-1250. 23. Yamasaki T, Kikuchi H, Higashi T, Yamabe H, Moritake K. Two surgically cured cases of subependymoma with emphasis on magnetic resonance imaging. Surg Neurol 1990; 33:329-335. 24. Hoeffel C, Boukobza M, Polivka M, et al. MR manifestations of subependymomas. AJNR Am J Neuroradiol 1995; 16:2121-2129. 25. Metwally, MYM: Textbook of neuroimaging, A CD-ROM publication, (Metwally, MYM editor) WEB-CD agency for electronic publication, version 11.2a. April 2010 26. Duncan JAI, Hoffman HJ. Intracranial ependymomas. In: Kaye AH, ed. Brain tumors. Edinburgh: Churchill Livingstone 1995:493- 504 27. Farwell JR, Dohrmann GJ, Flannery JT. Central nervous system tumors in children. Cancer. 1977;40(6):3123-3132 28. Mork SJ, Loken AC. Ependymoma: a follow-up study of 101 cases. Cancer. 1977;40(2):907-915 29. Schiffer D, et al. Histologic prognostic factors in ependymoma. Childs Nerv Syst. 1991;7(4):177-182 30. Schwartz TH, et al. Supratentorial ependymomas in adult patients. Neurosurgery. 1999;44(4):721-731 31. Kumar PV. Nuclear grooves in ependymoma. Cytologic study of 21 cases. Acta Cytol. 1997;41(6):1726-1731 32. Kawano N, Yada K, Yagishita S. Clear cell ependymoma. A histological variant with diagnostic implications. Virchows Arch A Pathol Anat Histopathol. 1989;415(5):467-472 33. Langford LA, Barre GM. Tanycytic ependymoma. Ultrastruct Pathol. 1997;21(2):135-142 34. Rosenblum MK, et al. Melanotic ependymoma and subependymoma. Am J Surg Pathol. 1990;14(8):729-736 35. Severi B, et al. Ependymoma of the foramen of Monro: ultrastructural characterization. Ultrastruct Pathol. 1989;13(1):35-42 36. Takeuchi H, et al. Epithelial differentiation and proliferative potential in spinal ependymomas. J Neurooncol. 2002;58(1):13-19 37. Rushing EJ, et al. Correlation of bcl-2, p53, and MIB-1 expression with ependymoma grade and subtype. Mod Pathol. 1998;11 (5):464-470 38. Ritter AM, et al. Ependymomas: MIB-1 proliferation index and survival. J Neurooncol. 1998;40(1):51-57 39. Verstegen MJ, et al. Proliferation- and apoptosis-related proteins in intracranial ependymomas: an immunohistochemical analysis. J Neurooncol. 2002;56(1):21-28 40. Sara A, Bruner JM, Mackay B. Ultrastructure of ependymoma. Ultrastruct Pathol. 1994;18(1–2):33-42 41. Park JP, et al. Constitutional de novo t(1;22)(p22;q11.2) and ependymoma. Cancer Genet Cytogenet. 1996;86(2):150-152 42. Urioste M, et al. Complex cytogenetic abnormalities including telomeric associations and MEN1 mutation in a pediatric ependymoma. Cancer Genet Cytogenet. 2002;138(2):107-110
