2007 ACJC Preliminary Examination, H2 Chemistry Syllabus 9647, Paper 3, Question 1
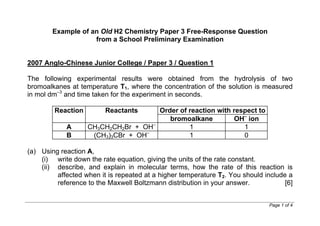
- 1. www.wewwchemistry.com Page 1 of 11 Example of an Old H2 Chemistry Paper 3 Free-Response Question from a School Preliminary Examination (The question stem is highlighted in green.) 2007 Anglo-Chinese Junior College / Paper 3 / Question 1 The following experimental results were obtained from the hydrolysis of two bromoalkanes at temperature T1, where the concentration of the solution is measured in mol dm–3 and time taken for the experiment in seconds. Order of reaction with respect toReaction Reactants bromoalkane OH– ion A CH3CH2CH2Br + OH– 1 1 B (CH3)3CBr + OH– 1 0 (a) Using reaction A, (i) write down the rate equation, giving the units of the rate constant.
- 2. www.wewwchemistry.com Page 2 of 11 Proposed Solution Rate equation : Rate = k[CH3CH2CH2Br][OH– ] Units for k : mol–1 dm3 s–1 (ii) describe, and explain in molecular terms, how the rate of this reaction is affected when it is repeated at a higher temperature T2. You should include a reference to the Maxwell Boltzmann distribution in your answer. [6] Proposed Solution When temperature of a reaction increases, average kinetic energy of reacting molecules increases. Number of reactant particles, per unit volume, with energy at least equal to the activation energy, Ea, increases. This increases the frequency of effective collisions. Since rate of reaction is proportional to the frequency of effective collisions, rate of reaction increases.
- 3. www.wewwchemistry.com Page 3 of 11
- 4. www.wewwchemistry.com Page 4 of 11 (b) Using reaction B, (i) calculate the time taken for the bromoalkane to decrease to 6.25 % of its original concentration, given that the half-life for this reaction is approximately 10.6 minutes. Proposed Solution Let n be the number of half-lives. ∴ Time taken for the bromoalkane to decrease to 6.25 % of its original concentration = 4 × 10.6 = 42.4 min
- 5. www.wewwchemistry.com Page 5 of 11 (ii) state and explain the effect on the rate of this reaction when the concentration of the hydroxide ion is doubled and the concentration of the bromoalkane is halved simultaneously. Proposed Solution Reaction is zero-order with respect to [OH– ]. ⇒ Rate of reaction is unaffected by changes to [OH– ]. Rate equation: Rate = k[(CH3)3CBr] ∴ Rate of reaction is halved.
- 6. www.wewwchemistry.com Page 6 of 11 (iii) The following mechanism is proposed for this reaction: Slow : (CH3)3CBr → (CH3)3C+ + Br– Fast : (CH3)3C+ + OH– → (CH3)3COH Draw a labelled enthalpy profile diagram for this proposed mechanism. [5] Enthalpy,H Reaction coordinate (CH3)3CBr + OH– (CH3)3COH+ Br– (CH3)3C+ + Br – + OH– Ea,2 Ea,1 !Hr
- 7. www.wewwchemistry.com Page 7 of 11 (c) The following observations were obtained when two halogenoalkanes react separately with aqueous silver nitrate. Halogenalkane Observations after adding AgNO3 (aq) CH3CH2CH2Br cream ppt forms after 2 minutes CH3CH2CH2I yellow ppt forms almost immediately Explain these observations as fully as you can. [3] Proposed Solution Reactions: i CH3CH2CH2X + H2O → CH3CH2CH2OH + H+ + X– (where X = halogen) ii Ag+ (aq) + X– (aq) → AgX(s) In the nucleophilic substitution of halogenoalkanes, the slow step involves the breaking of C–X. ⇒ Rate of this reaction depends on the strength of the C–X bond.
- 8. www.wewwchemistry.com Page 8 of 11 Since the C–Br bond is stronger than the C–I bond, the rate at which Br– (Reaction i) is released into solution to form cream precipitate of AgBr with Ag+ (Reaction ii) ions is slower than the rate at which I– is released into solution to form yellow precipitate of AgI. (d) Describe a simple chemical test that you could use to distinguish the following compounds. You are to include reagents and conditions, observations and a balanced equation(s) in each case. (i) and I II
- 9. www.wewwchemistry.com Page 9 of 11 Proposed Solution Test : To separate samples of compounds I and II, add Br2(aq). I II Observation Reddish brown bromine is decolourised, and a white precipitate is formed. Reddish brown bromine is not decolourised, and a white precipitate is not observed. Equation:
- 10. www.wewwchemistry.com Page 10 of 11 (ii) and III IV [6] [Total: 20] Proposed Solution Test : To separate samples of compounds III and IV, add KMnO4(aq) and H2SO4(aq) and heat. I II Observation Purple solution of KMnO4 is decolourised. Purple solution of KMnO4 is not decolourised.
- 11. www.wewwchemistry.com Page 11 of 11 Equations: Step 1: Hydrolysis of ester ! Step 2: Oxidation of primary alcohol