Food chemistry 2nd sem (full sylabus)
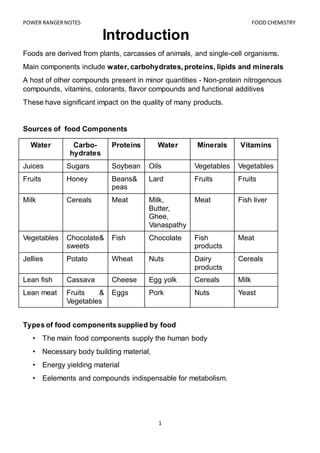
- 1. POWER RANGERNOTES FOOD CHEMISTRY 1 Introduction Foods are derived from plants, carcasses of animals, and single-cell organisms. Main components include water, carbohydrates, proteins, lipids and minerals A host of other compounds present in minor quantities - Non-protein nitrogenous compounds, vitamins, colorants, flavor compounds and functional additives These have significant impact on the quality of many products. Sources of food Components Water Carbo- hydrates Proteins Water Minerals Vitamins Juices Sugars Soybean Oils Vegetables Vegetables Fruits Honey Beans& peas Lard Fruits Fruits Milk Cereals Meat Milk, Butter, Ghee, Vanaspathy Meat Fish liver Vegetables Chocolate& sweets Fish Chocolate Fish products Meat Jellies Potato Wheat Nuts Dairy products Cereals Lean fish Cassava Cheese Egg yolk Cereals Milk Lean meat Fruits & Vegetables Eggs Pork Nuts Yeast Types of food components supplied by food • The main food components supply the human body • Necessary body building material, • Energy yielding material • Eelements and compounds indispensable for metabolism.
- 2. POWER RANGERNOTES FOOD CHEMISTRY 2 1. Body building material • Polysaccharides, proteins, and lipids serve as the building material of different structures of the plant and animal tissues used for food. • The structures made of these materials are responsible for the form and tensile strength of the tissues, and created the necessary conditions for metabolic processes to occur. • Compartmentalization resulting from these structures plays a crucial biological role in the organisms. • Some of the main components, as well as other constituents, are bound to different cell structures or are distributed in soluble form in the tissue fluids. 2. Energy yielding material Carbohydrate • The cheapest source of food. • Can be readily digested, absorbs and utilized for producing energy. • The most efficient source of energy. • Can furnish 50-70% of the total calorie intake. Carbohydrates are almost entirely derived from vegetable sources. • Main sources: Starch in the granular form in cereals, pulses and tubers and sugars that are present in milk, fruits and vegetables and sucrose. Protein • Oxidation of carbon skeletons of amino acids of proteins furnishes a minor but significant fraction of the daily energy requirement. Fat • Fat (Triglyceride) from plant and animal sources rank close behind carbohydrates as major source of energy. Fat has high fuel value. It has got high capacity to be stored as energy in the body. There is little difference between animal and vegetable fat as a source of energy.
- 3. POWER RANGERNOTES FOOD CHEMISTRY 3 3. Protective • Many of the minor components present food are nutritionally essential, such as vitamins and minerals. 4. OTHER FUNCTIONS IN FOOD 3. Other materials Many of the minor components originally present in the raw materials of food are nutritionally essential, such as vitamins and minerals. Others such as most free amino acids can be utilized by the body or impart desirable sensory properties to food products. Numerous groups, including tocopherols, ubiquinone, carotenoids, ascorbic acid, thiols, amines, and several other non-protein nitrogenous compounds serve as endogenous muscle antioxidants, playing an essential role in postmortem changes in meat. Some minor components are useless or even harmful if present in FUNCTIONS OF FOOD a. The main food components supply the human body with the necessary building material and source of energy, as well as elements and compounds indispensable for metabolism. b. Some plant polysaccharides are only partly utilized for energy. c. As dietary fiber they affect, in different ways, various processes in the gastrointestinal tract. d. The distribution of lipids in food raw materials depends on their role in the living animal and plant organisms. e. In an animal body, lipids occur primarily as an energy rich store of neutral fat in the subcutaneous adipose tissue; as kidney, leaf and crotch fat; as the intramuscular fat known as marbling; and as intramuscular or seam fat. • Many of the minor components originally present in the raw materials are nutritionally essential, such as vitamins.
- 4. POWER RANGERNOTES FOOD CHEMISTRY 4 • Some of them, although not indispensable, can be utilized by the body, including most free amino acids, or impart desirable sensory properties to food products. • Numerous groups, including tocopherols, ubiquinone, carotenoids, ascorbic acid, thiols, amines, and several other non-protein nitrogenous compounds serve as endogenous muscle antioxidants, playing an essential role in postmortem changes in meat. • Some minor components are useless or even harmful if present in excessive amounts. • Most food raw materials are infected with different microorganisms, putrefactive and often pathogenic, and some contain parasites and the products of microbial metabolism. • A number of compounds are added intentionally during processing, to be used as preservatives, antioxidants, colorants, flavorings, sweeteners, and emulsifying agents, or to fulfill other technological purposes. WATER • The content of water in various foods ranges from a few percent in dried commodities (dried milk) to 90% in many fruits and vegetables • about 15% in grains • 16 to 18% in % in butter • 20% in honey • 35% in bread, • 65% potato and cassava • 75% in meat and fish • 90% in many fruits and vegetables Carbohydrates • Carbohydrates are widely distributed in plant and animal tissues. • The carbohydrates commonly occurring in foods are starch, glucose, fructose, sucrose and lactose. About 50-70 % of energy value in the average diet is provided by carbohydrates.
- 5. POWER RANGERNOTES FOOD CHEMISTRY 5 • They are the cheapest source of energy. Glucose derived by digestion of carbohydrates is stored as glycogen in liver and muscle tissues and used as the main source of energy in the body. • Hence food must always contain adequate amounts of carbohydrates. Important sources of carbohydrates • The important sources of carbohydrates in the diets of children and adults are cereals, millets, roots, tubers, pulses, sugar and jaggary, while milk and sugar are important sources in the diets of infants. Carbohydrate content of some important food Name of food Carbohydrate g/100g Cereals and millets (rice, jowar, etc.) 63 – 79 Pulses (Bengal gram, red gram, etc.) 56 – 60 Nuts and oilseeds 10 – 25 Roots and tubers (Potato, tapioca, sweet potato, etc.) 22 – 39 Arrow root flour 85 – 87 Cane sugar 99 Sago 87 – 89 Honey 79 – 80 Jaggery 94 – 95 Milk (fluid) 4 – 5 Dried fruits (Raisin, dates, etc.) 67 – 77 Fresh fruits 10 - 25 Protein • The protein content in foods is present mainly as crude protein (i.e., as Nx6.25).
- 6. POWER RANGERNOTES FOOD CHEMISTRY 6 • The nitrogen-to-protein conversion factor (N:P) of 6.25 has been recommended for most plant and animal food products under the assumption that the N content in their proteins is 16% and they do not contain non-protein N. • The N content in the proteins in various food, however, is different because it depends o the amino acid component of protein compounds, such as free peptides and amino acids, nucleic acids and their the non-protein N may constitute up to 30% of total N. • In many of these compounds the C:N ratio is similar to the average in amino acids Protein contents of different groups of foods Food groups Protein content % Cereals and millets 6.14 Pulses (legumes) dry 18-24 Oilseeds and nuts (except coconut) 18-40 Meat, fish and liver 18-20 Eggs 12-14 Milk (fresh) 3.5-4.0 Milk, dried whole 26-28 Milk, dried, skimmed 33-38 Vegetables, fresh Leafy 1-4 Roots and tubers 1-1.5 Other vegetables 1-7 Lipids • A group of naturally occurring substances characterized by their insolubility in water and solubility in organic solvents. They occur in plant and animal tissues.
- 7. POWER RANGERNOTES FOOD CHEMISTRY 7 • They include simple lipids, compound lipids and derived lipids. • Oils and fats serve as the main source of energy. • They also provide the essential fatty acids. They are good source of fat soluble vitamins. • Fat serve as an insulating material in the subcutaneous tissues and around vital organs. • It provide materials for the synthesis of cholesterol and certain hormones. • Lipoproteins and glycoproteins are essential for maintaining cellular integrity. • Fat is essential for maintaining good health Fat contents of different groups of foods Food groups Fat content % Fruits, vegetables <1 Lean fish muscle <1 Oilseeds and nuts (except coconut) 98 Beef ,Meat, 6 Egg yolk 32 Milk (fresh) 3.5-4.0 Butter 85 Minerals • Minerals are naturally occurring inorganic element in the soil which is transformed into an organic compound for use and assimilation by the human body.
- 8. POWER RANGERNOTES FOOD CHEMISTRY 8 • There are 16 minerals that the human body needs in order to function properly. • Most of the important minerals are easily supplied in common foods. • Fruits, vegetables, and cereals are the chief sources of mineral elements in diet. • Milk products supply the majority of calcium and phosphorus in the diet. Factors affecting food composition a) Raw Materials i. The content of different components in food raw materials depends on - the species and variety of the animal or plant crop; - on the conditions of life, and age of the farm harvesting of the plants; - on the feeding, conditions of life, and age of the farm animals or - the fishing season for fish and marine invertebrates; and - on post harvest changes that take place in the crop during storage. ii. The food industry, by establishing quality requirements for raw materials, can encourage producers to control, within limits, the contents of the main components in their crops; e.g: starch in potatoes, fat in various meat cuts, pigments in fruits and vegetables and in the flesh of fish from aquaculture, or protein in wheat and barley, as well as the fatty acid composition of lipids in oilseeds and meats. iii. The contents of desirable minor components can also be effectively controlled for example, the amount of natural antioxidants to retard the oxidation of pigments and lipids in beef. iv. Contamination of the raw material with organic and inorganic pollutants can be controlled by observing recommended agricultural procedures in using fertilizers, herbicides, and insecticides, and by seasonally restricting certain fishing areas to avoid marine toxins. v. The size of predatory fish like swordfish, tuna or shark which are fished commercially can be limited to reduce the risk of excessive mercury and arsenic in the flesh. ii) Processed foods
- 9. POWER RANGERNOTES FOOD CHEMISTRY 9 • The composition of processed foods depends on the recipe applied and on changes taking place due to processing and storage. • These changes are mainly brought about by endogenous and microbial enzymes, active forms of oxygen, heating, chemical treatment, and processing at low or high pH. Changes occurring in food due to processing 1. Leaching of soluble, desirable and undesirable components, such as vitamins, minerals and toxins during washing, blanching and cooking. 2. Dripping after thawing or due to cooking 3. Loss of moisture and volatiles due to evaporation and sublimation 4. Absorption of desirable or harmful compounds during salting, pickling, seasoning, frying or smoking. 5. Formation of desirable or harmful compounds due to enzyme activity, such as the development of typical flavor in cheese or decarboxylation of amino acids in fish marinades. 6. Generation of desirable or objectionable products due to interactions of reactive groups induced by heating or chemical treatment, such as flavors or carcinogenic compounds in roasted meats, or trans-fatty acids in hydrogenated fats. 7. Formation of different products of oxidation of food components, mainly of lipids, pigments, and vitamins 8. Loss of nutrients and deterioration of dried fish due to attacks by flies, mites and beetles. 2. WATER IN FOODS Introduction Water is the most critical of all nutrients. It is an essential constituent of all cell structures and is the medium in which all the chemical reactions of a cellular metabolism take place. Water is the major component of all living organisms. It constitutes 60% or more of the weight of most living things, and it pervades all portions of every cell. Water is the universal solvent and dispersing agent, as well as a very reactive chemical compound.
- 10. POWER RANGERNOTES FOOD CHEMISTRY 10 Biologically active structures of macromolecules are spontaneously formed only to aqueous media. Intracellular water is a medium in which structural arrangement and all metabolic processes occur. It is an active partner of molecular interactions, participating directly in many biochemical reactions as a substrate or a product. Its high heat capacity allows water to act as a heat buffer in all organisms. Regulation of water contents is important in the maintenance of homeostasis in all living systems. Stability, wholesomeness, and shelf life are significant features of foods that are, to a large degree, influenced by the water content. Dried foods were originally developed to overcome the constraints of time and distance before consumption. Canned and frozen foods were developed next. The physical properties, quantity, and quality of water within food have a strong impact on food effectiveness, quality attributes, shelf life, textural properties and processing. Water is the major component of many foods. Its quantity, location and orientation profoundly influence the structure, appearance and taste of foods. Stability, wholesomeness, and shelf life are significant features of foods that are, to a large degree, influenced by the water content. Fresh foods contain large quantity of water and hence effective forms of preservation is needed for long time storage.Water content of different groups of foods is presented in table 2.1.1 Removal of water by drying or converting it into ice crystals by freezing greatle alters the native properties of foods. The physical properties, quantity, and quality of water within food have a strong impact on food effectiveness, quality attributes, shelf life, textural properties and processing. Table 2.1.1 Water content of different groups of foods Food Water content % Fruits Apple,grapes,oranges 90
- 11. POWER RANGERNOTES FOOD CHEMISTRY 11 Pears 80-85 Tomato, strawberries 90-92 Vegetables Banana, peas 73-80 Beet root, potatoes,carrots 86-94 Cabbage, cauliflower, lettuce 90-95 Meat Fish 65-84 Beef/Mutton 55-70 Chicken 65-75 Pork 55-60 Structure and Properties of water • Water is a familiar material, but it has been described as the most anomalous of chemical compounds. • Although its chemical composition, HOH, or H2O, is universally known, the simplicity of its formula belies the complexity of its behavior. • Its physical and chemical properties are very different from compounds of similar complexity, such as HF and H2S. • Although a water molecule is electrically neutral as a whole, it has a dipolar character. • The high polarity of water is caused by the direction of the H-O-H bond angle, which is 104.5o , and by an asymmetrical distribution of electrons within the molecule. O / 104.5° + H H+ • In a single water molecule, each hydrogen atom shares an electron pair with the oxygen atom in a stable covalent bond. • However, the sharing of electrons between H and O is unequal because the more electronegative oxygen atom tends to draw electrons away from the hydrogen nuclei. • The electrons are more often in the vicinity of the oxygen atom than in the vicinity of the hydrogen atom.
- 12. POWER RANGERNOTES FOOD CHEMISTRY 12 • The result of this unequal electron sharing is the existence of two electric dipoles in the molecule, one along each of the H-O bonds. • The oxygen atom bears a partial negative charge and each hydrogen atom a partial positive charge. Because the molecule is not linear, H-O-H has a dipole moment. • Water molecules can interact through electrostatic attraction between the oxygen atom of one water molecule and the hydrogen of another. Type of hydrogen bond in water • Such interactions, which arise because the electrons on one molecule c an be partially shared with the hydrogen on another, are known as hydrogen bonds. • The H2O molecule, which contains two hydrogen atoms and one oxygen atom in a nonlinear arrangement, is ideally suited to engage in hydrogen bonding. • It can act both as a donor and as an acceptor of hydrogen. • The nearly tetrahedral arrangement of the H orbital about the oxygen atom allows each water molecule to form hydrogen bonds with four of its neighbors. • An individual, isolated hydrogen bond is very labile. It is longer and weaker than a covalent O-H bond. • The hydrogen bond’s energy, that is, the energy required to break the bond, is about 20kJ/mol. • These bonds are intermediate between those of weak Van der Waals interactions (about 1.2 kJ/mol) and those of covalent bonds (460kJ/mol). • Hydrogen bonds are highly directional; they are stronger when the hydrogen atom and the two atoms that share it are in a straight line. • Hydrogen bonds are not unique to water. • They are formed between water and different chemical structures, as well as between other molecules (Intermolecular) or even within a molecule (Intramolecular Water in foods • Most natural foods contain water up to 70% of their weight. • Water in foods is classified in to two types: (a) bound water and (b) free water
- 13. POWER RANGERNOTES FOOD CHEMISTRY 13 • Water that can be extracted easily from foods by squeezing or cutting or pressing is called as free water a) Bound Water Water that is held so tightly by another molecule (usually a large molecule such as a protein) that it no longer has the properties of free water; water that is not easily removed from the food is called bound water. • This water is not free to act as solvent for salts and sugars. • It can be frozen only at very low temperatures. It exhibits no vapour pressure. Its density is greater than water. • The water molecules are bound to polar groups or ions on molecules such as starches, pectin, and proteins. • This water is held firmly. • The subsequent water layers are held less firmly. The bound water is of three types i. Constitutional ii. Vicinal iii. Multilayer • i. Constitutional: They form an integral part of a non aqueous constituent forming <0.03%. It is constituted by a monolayer of water molecules absorbed on the polar absorption site of the molecule is almost immobilized and thus behaves, in many respects, like part of the solid or like water in ice. • ii. Vicinal: It is the bound water that strongly acts with specific hydrophilic sites of non-aqueous constituents to form a monolayer coverage; water-ion and water-dipole bonds forming 0.1 to 0.9%. • iii. Multilayer: Bound water that forms several additional layers around hydrophilic groups, water-water and water-solute hydrogen bonds. It forms 1- 5%.
- 14. POWER RANGERNOTES FOOD CHEMISTRY 14 b) Free or entrapped water • Water that can be extracted easily from foods by squeezing or cutting or pressing is called as free water. • Flow is unimpeded; properties close to dilute salt solutions. • Free water is held within matrix or gel, which impedes flow forming 5-96%. • Entrapped water is immobilized in capillaries or cells but if released during cutting or damage, it flows freely. Water activity • Water activity or aw is a measurement of water content. • It is defined as the vapour pressure of a liquid divided by that of pure water at the same temperature; therefore, pure distilled water has a water activity of exactly one. aw=P/P0 • where p is the vapor pressure of water in the substance, and P0 is the vapor pressure of pure water at the same temperature. • As the temperature increases, aw typically increases, except in some product with crystalline salt or sugar. • Higher aw substances tend to support more microorganism. • Bacteria usually require at least 0.91, and fungi at least 0.7. • Many of the chemical and biological processes that cause deterioration of foods, and ultimately spoilage, are water dependent. • Water activity aw represents the water which is made available for the microbial action. • Microbial growth is directly linked to water activity. • Essentially, water activity is the measure of the degree to which water is bound within the food, and hence is unavailable for further chemical or microbial activity • Relative humidity of moist air is defined in the same way except that by convention, relative humidity is reported as a percentage whereas water activity is expressed as a fraction. • Thus if a sample of meat sausage is sealed within an airtight container, the humidity of the air in the head space will rise and eventually equilibrate to a relative humidity of, say 83%, which means that the water activity (aw) of the meat sausage is 0.83.
- 15. POWER RANGERNOTES FOOD CHEMISTRY 15 Water activity and Shelf life of Foods • It is an important consideration for food product design and food safety. • Food designers use water activity to formulate products that are shelf stable. • If a product is kept below a certain water activity, then mold growth is inhibited. This results in a longer shelf-life. • Water activity is used in many cases as a critical control point for Hazard Analysis and Critical Control Points (HACCP) programs. • Samples of the food product are periodically taken from the production area and tested to ensure water activity values are within a specified range for food quality and safety. • Measurements can be made in as little as five minutes, and are made regularly in most major food production facilities. Water activity of some foods Substance aw Distilled Water 1 Tap water 0.99 Raw meats 0.99 [ Milk 0.97 Juice 0.97 Cooked bacon < 0.85 Saturated NaCl solution 0.75 Point at which cereal loses crunch 0.65 Dried fruits 0.60 Typical indoor air 0.5 - 0.7 Honey 0.5 - 0.7 Dried fruit 0.5 - 0.6 Microbial growth • Many of the chemical and biological processes that cause deterioration of foods, and ultimately spoilage, are water dependent. • Microbial growth is directly linked to water activity.
- 16. POWER RANGERNOTES FOOD CHEMISTRY 16 • No microbes can multiply at a water activity below 0.6. Dehydration • Dehydration is arguably the oldest form of food preservation; • The sun drying of meat and fish has been traces to the beginning of recorded history. • Drying relies on removing water, thus making it unavailable for microbial growth. Salting or curing • Salting or curing has the same effect. • A saturated solution of common salt has a water activity of close to 0.75. • Thus by adding sufficient salt to foods, the water activity can be lowered to a level where most pathogenic bacteria are inactivated but the moisture content remains high. • Intermediate moisture content foods (IMF), such as pet food and continental sausages; rely on fats and water-binding humectants such as glycerol to lower water activity. • Fat, being essentially hydrophobic, does not bind water, but acts as filler for IMF to increase the volume of the product. • The water activity of the salted food is 0.8. Benefits of drying of food • None of the dangerous pathogenic bacteria associated with food, such as Clostridium or Vibrio spp. which cause botulism and cholera, can multiply at water activity values below about 0.9. • Drying or providing sufficient water-binding humectants is an effective method of preventing the growth of food-poisoning bacteria. • Only osmophilic yeast and some molds can grow at water activities in the range 0.6 to 0.65. • Thus, by reducing the water activity below these values, foods are microbial stable. • That is, unless the packaging is such that the food becomes locally rewet, in which case local spoilage can occur, for example, when condensation occurs within a hermetically sealed package subject to rapid cooling.
- 17. POWER RANGERNOTES FOOD CHEMISTRY 17 Chemical reactions and water activity • Various chemical reactions that proceed, and may be accelerated, at low values of water activity. • Maillard reactions leading to lysine loss and brown color development peaks at aw around 0.5 to 0.8. • Nonenzymatic lipid oxidation increases rapidly below aw = 0.4. • Enzymatic hydrolysis decreases with water activity down to aw = 0.3 and is then negligible. • Water is facilitator of biochemical deterioration of foods. • Dry foods are much more stable than wet foods, because any water remaining to them has low activity, aw. • Freezing removes water from the food matrix by forming ice crystals. • Although the ice crystals remain in the food, the remaining water which is in contact with the food matrix becomes concentrated with solutes and it’s aw becomes low. • Freezing is therefore akin to drying and this is the rationale for preserving food by freezing. • Most micro-organisms cease functioning below the water activity of about 0.7. FOOD LIPIDS AND FISH LIPID Fat/ lipids of Food • Fat is a generic term for a class of lipids. • Fats are produced by organic processes in animals and plants. • These are extracted and used as an ingredient. • All fats are insoluble in water and have a density significantly below that of water (i.e. they float on water.) • Fats that are liquid at room temperature are often referred to as oil. • Most fats -composed primarily of triglycerides; • some monoglycerides and diglycerides are mixed in products with a lot of saturated fats tend to be solid at room temperature. • Products containing unsaturated fats, which include monounsaturated fats and polyunsaturated fats, tend to be liquid at room temperature.
- 18. POWER RANGERNOTES FOOD CHEMISTRY 18 Types of fat • Predominantly saturated fats (solid at room temperature) • All animal fats (e.g. milk fat, lard, tallow), as well as palm oil, coconut oil, cocoa fat and hydrogenated vegetable oil (shortening). • Predominantly unsaturated and remain liquid at room temperature • Vegetable fats- from olive, peanut, maize (corn oil), cottonseed, sunflower, safflower, and soybean, are. • However, both vegetable and animal fats contain saturated and unsaturated fats. • Some oils (such as olive oil) contain in majority monounsaturated fats, while others present quite a high percentage of polyunsaturated fats (sunflower, rape). Saturated fats If the fatty acid has all the hydrogen atoms it can hold it is said to be saturated (see below) H H H H H │ │ │ │ │ C C C C C │ │ │ │ │ H H H H H This type of fat is typically found in large amounts in foods from animals, e.g. meat, butter, cheese and cream. Many baked goods such as cakes, biscuits and pastries are also high in saturated fat. Excessive intake of saturated fat can increase blood cholesterol levels. Unsaturated fats
- 19. POWER RANGERNOTES FOOD CHEMISTRY 19 • In unsaturated fats, some of the carbon atoms are joined to others by a double bond and, therefore, could accept more hydrogen atoms. • They are not completely saturated with hydrogen, so are called unsaturated fats. H H H H H │ │ │ │ │ C C ═C C C │ │ │ H H H There are two main types of unsaturated fats– 1. Monounsaturated (containing one double bond) and 2. Polyunsaturated (containing more than one double bond). Most monounsaturated and polyunsaturated fats have good qualities, with one exception - trans-fatty acids. Trans fatty acids are, an unsaturated fat but offer no health benefits. Monounsaturated fatty acids There is one double bond is present Found in significant amounts in most types of fats of plant origin, such as nuts, avocado pears, rapeseed oil and olive oil. Monounsaturated do not raise blood cholesterol and evidence shows that they reduce blood cholesterol levels if they replace saturated fat in the diet • Oleic acid is the main monounsaturated fat in our diets and this is sometimes called omega-9 (because the double bond is in position 9 of the fatty acid chain). • Found in significant amounts in most types of nuts, avocado pears, rapeseed oil and olive oil and spreads made from these.
- 20. POWER RANGERNOTES FOOD CHEMISTRY 20 Polyunsaturated fatty acids • There is more than one double bond, • These come mostly from vegetable sources, such as sunflower oil or seeds, but are also found in, nuts, green leafy vegetables and oily fish such as mackerel and sardines. • Polyunsaturated fatty acid can actively reduce blood cholesterol levels. • The polyunsaturated found in oily fish specifically appear to have no effect on blood cholesterol levels, but they do alter the consistency of blood. • There are two 'series' of polyunsaturated fats in food • They are also known as essential fatty acids. They are omega 3 and omega 6. • Essential fatty acids are so called because the body cannot make them but they are essential to the body's normal functioning, therefore, must be supplied through diet. • Humans are unable to make these essential fatty acids, because they do not have the particular destaurase enzymes that insert double bonds in position 3 and 6 of the fatty acid chain • Fats also carry the fat soluble vitamins A, D, E and K. • Fats and lipids are energy storage materials in plants and animal tissues. Functions of lipid 1. Important sources of metabolic energy (ATP) • Lipids are the most energy rich of all classes of nutrients: • Gross energy value of lipid 9.5 Kcal/g, protein 5.6 Kcal/g, carbohydrate 4.1 Kcal/g and the net values were 9 K.cal /g, 4.0K.cal/g and 4.k.cal/g for fat, protein and carbohydrate respectively. • Dietary lipids may be used to spare the more valuable fo protein r growth. • The free fatty acids derived from triglycerides (fats and oils) are the major aerobic fuel sources for energy metabolism. 2. Forms part of membrane Lipids are essential components of all cellular and subcellular membranes (polyunsaturated fatty acids containing phospholipids, and sterol esters). 3. Serve as biological carriers:
- 21. POWER RANGERNOTES FOOD CHEMISTRY 21 • For the absorption of the fat soluble vitamins A, D, E and K. 4. Source of essential fatty acids: • Linoleic, Linolenic and Arachidonic acids- which in turn are essential for the maintenance and integrity of cellular membranes, are required for optimal lipid transport (bound to phospholipids as emulsifying agents) • Precursors of the prostaglandin hormones. 5. Mechanical cushion/support: Play a role as mechanical cushion/support for the vital body organs, and aid in the maintenance of neutral buoyancy. 6. Source of essential steroids: • Needed to perform a side range of important biological functions. • Sterol cholesterol is involved in the maintenance of membrane systems, for lipid transport, • A precursor of vitamin D3, the bile acids, and the steroid hormones – androgens, estrogens, adrenal hormones, and corticosteroids. 7. Food flavor / mouth feel: • They play a role in food flavor / mouth feel, palatability, texture and aroma. Fish lipids • Lipid in fish generally carries natural flavour components and provides and preserves other generated during cooking, pickling or other processing. • A certain amount of fat and fatty acid assists in providing smoothness of texture during mastication of lean fish. In fatty fish the influence of fat on texture is even more important. • The lipid content of fish varies widely from species to species and even within the same species from one individual to another depending on age, sex, environment and season. Lipid content of seafood Type of fish Fat %
- 22. POWER RANGERNOTES FOOD CHEMISTRY 22 Fatty fish 10.0 Lean fish 0.5 Crustaceans 2.1 Mollusks 1.5 Distribution of Fat in Fish • The term lipid will be used for total fat component in fish. • However, term fat is used for selected anatomical deposits, which are mostly triglyceride. • In lean fish the dark (red or lateral line) muscle has about twice the lipid of white muscle. • The percentage of cellular lipid in the white muscle is normally altered by season. • The lean muscle fish generally have more fat in livers (e.g. cod) which show seasonal variation. • In the fatty fish species, the muscle shows fluctuating levels of seasonal variation in neutral fat a. Neutral fat (Triglycerids) • Fforms the major constituent of fish lipid. • There are variations in the amount of neutral fat in muscle. • The belly flap is a high fat section of many fishes. (E.g. In mackerel -29% lipid in belly flaps, 18.3% lipid in dark muscle and 7.6% in while muscle). • In male mackerel the skin fat forms 40% of the total fat in the whole fish. • Triglyceride distributed through fish muscle tends to have a homogeneous fatty acid composition Most species of marine organisms try to obtain an optimal fat and fatty acid composition and their behavior and food preferences lead towards this objective. b. Basic cellular lipids (Phospholipids) • Lean white fish muscle contain a minimum of about 0.7% of basic cellular lipid,- 85-95% is ‘polar’ lipids, mostly phosphatidyl ethanolamine and phosphatidyl choline. • The balance of this type of basic lipid includes sterol ester and free sterol, free fatty acidsand triglyceride.
- 23. POWER RANGERNOTES FOOD CHEMISTRY 23 • This basic mixture represented the structural lipid of cell walls, and that any excess of triglyceride and/or certain other non-polar lipids such as wax esters or glyceryl ethers, provided the ‘fat’ of fatty fish. c. Fatty acids of fish lipid Classification of fish lipid fatty acids 1. Saturated acids 2. Monoenoic acids 3. Polyenoic acids. The fatty acid s present in fish lipid is classified into three groups, saturated acids, monoenoic acids and polyenoic acids. 1. Saturated fatty acids Myristic or tetradecanoic (14:0) - 5-10% of the total Palmitic or hexadecanoic(16:0) - 10-30% of the total Stearic or octadecanoic(18:0). - 1-3% of of the total Longer fatty acids (20:0, 22:0 and 24:0) - 0.01-0.1%. These fatty acids can all be biosynthesized by the organisms, but they are also freely absorbed from dietary fats. Odd number straight-chain fatty acids: If Propionate molecule primes the two- c arbon fatty acids chain extension process instead of an acetate molecule- odd- chain fatty acids are also formed. Bacteria also contributethese fatty acids The fatty alcohols of copepod esters also have C15 and C17 methyl-branched and odd carbon structures which are probably oxidized to corresponding acids and then deposited in the fats of capelin, mackerel and herring. 2. Monoenoic Fatty acids Monoenoic acids offish lipids Oleic C18:1 or 9-octadecenoic (1ω7), Gadoleic or 11-eicosenoic (20:1 ω9) and Cetoleic or 11-docosenoic (22:11 ω1). Palmitoleic acid (16:1) and oleic (18:1 ω9) of fish oils can be synthesized by fish and other marine organisms from acetate units.
- 24. POWER RANGERNOTES FOOD CHEMISTRY 24 Palmitoleic acid can be carbon extended to cis-vanccenic acid (18:1 ω7), which forms 10-30% of the total 18:1 isomers. 20:1 ω9 is by chain elongation of 18:1 ω9. 20:1 ω11 and 20:1 ω7 ,20:1 ω9 –occur in several fish oils. In pacific herring oil 20:1 ω11 –Higer proportions. Dominant 22:1 isomer in marine fish oil is 22:1 ω11. In herring only the 22:1 ω9 isomer is biosynthesized. 3. PolyenoicFatty acids • Important polyenoic acid present in fish lipids • Eicosapentaenoic (EPA) (C20:5 ω3) • Dohosahexaenoic (DHA) (C22:6 ω3). • These fatty acids give marine oils their most specific characteristics. • They originate in unicellular phytoplankton or in some seaweed. • The average fatty acids composition of phytoplankton includes all the principal fo fatty acids und in the oils and lipids of the higher organisms. • The two common ‘plant’ C18 fatty acids 18:2 ω6 (linoleic acid) and 18:3 ω3 (linolenic acid) –Not more than 1% or 2% of fatty acids. • Many invertebrate lipids-20:5 ω3 is the dominant polyunsaturated fatty acids - possibly due to dietary algae. d. Wax Easters and Glyceryl Ethers Wax esters of marine organisms Two main classes: (1) those rich in 16:0 acids (2) those rich in 22:1 acids. The chain length of fatty acids: In the range C32 to C36. The copepod wax ester rich in 22:1 alcohol, has a high content of 14:0 acids. They also have a high proportion of 16:0 fatty alcohols in their wax esters as part of their body lipid.
- 25. POWER RANGERNOTES FOOD CHEMISTRY 25 The ‘castor oil’ fish Ruvettus pretiosus contains wax esters, which are responsible for the purgative effect. Properties of fat (a) The fats are insoluble in water, but readily soluble in ether, chloroform, benzene, carbon tetra chloride (b) They are readily soluble in hot alcohol but slightly soluble in cold. (c) They are themselves good solvents for other fats, fatty acids etc. (d) They are colourless, odourless, tasteless and neutral in reaction. (e) Several neutral fats are readily crystallised, e.g. mutton, beef (f) Their melting points are low. (g)The specific gravity is about 0.86. Hence the fatreadily float in water. They spread uniformly over the surface of wates and this spreading effect is to lower surface tension. Functions of lipoprotein The various types of lipoproteins have different functions. Chylomicrons and VLDLs: Chylomicrons and VLDLs are the principal carriers of triglycerides and Choleterols in blood.The concentrations are increased in atheroscorosis and coronary thrombosis. LDLs:In LDLs the predominant lipid is cholesterol and phospholipids. Increased in atherosclerosis and coronary thrombosis, etc. HDLs: HDLs are predominant lipid is phospholipid and proteins. LDLs and HDLs are involved in the cholesterol transport. LDLs carry about 80% of cholesterol while the remaining is carried by HDLs. LDLs carry cholesterols to cells for their use where as HDLs carry excess cholesterol away from the cells to the liver for processing and excretion from the body. The levels of LDL correlate directly with heart disease, where as HDL levels correlates inversely with heart disease risk. Thus HDL is some times referred to as “good” cholesterol and LDL as “bad” cholesterol.
- 26. POWER RANGERNOTES FOOD CHEMISTRY 26 Role of Fish Lipids in Human Nutrition To achieve a balanced diet there is a need to reduce total fat intake and it is also important to make sure that the type of fat we eat is right. The fat that is not beneficial to human is the hard "saturated" fat which comes mainly from the fat of land animals such as cows and sheep. Fish fat has several beneficial effects. 1. Providefood with low fat • Fish is a good food for a low fat diet. It is low in calories and many types of fish do not contain any unsaturated fat. • The nutritional value of fish will vary slightly according to the location it is harvested, the cut of fish, and the age of the fish. • The method used for cooking will have an affect on it also. 2. Reduce the cholesterollevelin the blood • Cholesterol is the type of fat which is naturally produced by our bodies and is also found in the diet. • It is usually deposited in the lining of the blood stream vessels, causing them to narrow. • The heart then has to work harder to pump blood around the body. • Blood clotting can result and the cholesterol deposits can be very hard. • Tissues can become deprived of oxygen when the blood vessels become blocked. • Unsaturated fats can help to reduce the cholesterol level in the blood, thus lowering the risk of heart disease. • Oil-rich fish such as mackerel, sardines, herring and sprats are rich in unsaturated fats containing Omega-3 so valuable for health. 3. Source Omega-3 fatty acids • Two fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic (DHA), collectively known as Omega-3, are essential fatty acids. • Schizophrenia symptoms can be eliminated or at least vastly diminished by oral supplementation with EPA.
- 27. POWER RANGERNOTES FOOD CHEMISTRY 27 • DHA is the building block of human brain tissue and is particularly abundant in the grey matter of the brain and the retina. • Low levels of DHA have been associated with depression, memory loss, dementia and visual problems. • DHA is particularly important for fetuses and infants; the DHA contents of the infant’s brain triples during the first three months of life. • Optimal levels of DHA are therefore crucial for pregnant and lactating mothers. • Oil-rich fish, such as salmon, trout, mackerel, herring and sardines, are an excellent source of Omega-3 fatty acids, which are essential to our diet. • Eating oil-rich fish provides the Omega-3 fatty acids needed for the body. • Omega-3 oils from fish have a lowering effect on blood fats. • This decreases the chance of the blood vessels clogging with cholesterol. • Omega-3 can also make blood less "sticky", and it therefore flows more easily around the body. This can reduce the risk of a heart attack. • They also help to reduce blood pressure a little and keep the heart beat steady. Omega-3 oil in fish can reduce the risk of dying from heart attacks. 4. Fish oils preventcancer • Fish oils can help to prevent cancer cells progressing to the tumor stage. • They may also reduce inflammation and provide relief for people suffering from rheumatoid arthritis and even some skin disorders such as psoriasis. 5. Needed for the developmentof brain • Omega-3 oils can play an important part in aiding the development of brain. • Expectant mothers are advised to eat a lot of oil-rich fish in the last three months of pregnancy to assist the baby's brain growth. • A good supply of Omega-3 oils assists the development of nerves and eyesight. OXIDATION OF OIL/FAT Lipid oxidation is one of the major causes of food spoilage. In edible oils and fat-containing foods, it leads to the development of various off flavors and off odors, generally known as oxidative rancidity, which renders the foods less acceptable. It may also be able to decrease the nutritional value of food and in some cases may produce potentially toxic products. It may be sometimes desirable as in the case of cheese.
- 28. POWER RANGERNOTES FOOD CHEMISTRY 28 Oxidation is caused by a biochemical reaction between fats and oxygen called as autoxidation and it is the main reaction involved. The lipids of foods can be oxidized by (i) non enzymic and (ii) enzymic mechanisms. Lipid oxidation generally occurs after a long induction period. Once started it is generally a very rapid reaction. (i) Non enzymic oxidation • Non enzymatic lipid oxidation (Autoxidation) proceeds by a free radical mechanism. • It is catalysed by light and free radical-producing substances and yields hydroperoxide (ROOH). • This primary product is relatively unstable. • It enters into numerous reactions involving substrate degradation and interaction, resulting in numerous compounds of various molecular weigh, flavour generating and biological significance. Free radical mechanism A free radical is a compound with an odd number of unpaired electrons. H H H — C —C — C — H H H ↓ H H H — C —C• — C — + H• H H When initiated two free radicals are formed. These radicals are very reactive and generally do not have long life time. Lipid oxidation There are three main steps
- 29. POWER RANGERNOTES FOOD CHEMISTRY 29 i. Initiation ii. Propagation iii. Termination i. Initiation The initiation occurs by direct attack of oxygen in its most stable form on double bonds of fatty acids (RH). The presence of a double bond in the fatty acid (RH) weakens the C-H bonds on the carbon atom adjacent to the double bond and so makes H removal easier. Oxygen attack at the end carbon of the double bond and forms hydrogen peroxide. Hydroperoxides breakdown in several steps to form free radicals. Initiator RH +O2 → ROOH → Free radicals (R• , ROO • ) • The carbon radical tends to be stabilized by a molecular rearrangement to form a conjugated diene. • Oxygen attack the end carbon of the double bond and forms hydrogen peroxide. • Hydroperoxide breakdown, in several steps, to form free radicals. ii. Propagation • Once the initial radicals have formed, the formation of other radicals proceeds rapidly. • The new radicals will not be at the double bond. To remove a hydrogen from a double bond requires 80 Kcal/mole. • To remove a hydrogen alpha to a double bond only requires 15 Kcal/mole. • As a peroxyl radical is able to abstract H from another lipid molecule (adjacent fatty acid), especially in the presence of metals such as copper or iron, thus causing an autocatalytic chain reaction. • The peroxyl radical combines with H to give a lipid hydroperoxide (or peroxide). • This reaction characterizes the propagation stage.
- 30. POWER RANGERNOTES FOOD CHEMISTRY 30 • The peroxyl radical combines with H to give a lipid hydroperoxide (or peroxide). • This reaction characterizes the propagation stage. • R • . + O2 → ROO • . • • ROO• . + RH → ROOH + R • . iii) Termination Any kind of alkyl radicals (lipid free radicals) R• can react with a lipid peroxide ROO• to give non-initiating and Non-propagating species such as the relatively stable dimers ROOR or two peroxide molecules combining to form hydroxylated derivatives (ROH). R• + R• → R-R R • + ROO• → ROOR 2ROO• → ROO-OOR Effects of Lipid Oxidation in Foods • When lipids in food are oxidised, some of the product formed impart odour and flavours, usually undesirable, to the food. • The free radicals generated during the oxidation reaction and some of the molecules formed when oxidized compound decombos (aldehydes, acids, alcohols, ketones etc.) can interact with and alter other constituents including pigments, vitamins, proteins and amino acids. • These interactions can result in colour, texyure and nutritive value. • If foods containing oxidized lipids are consumed, the oxidation products could be involved in reactions leading to pathological changes. • For e.g. malonaldehyde and oxidation product of certain polyunsaturated fatty acids found in many foods, is a potential carcinogen. (ii) Enzyme catalysed lipid oxidation • Enzyme reaction starts with the action of lipolysis. • Released polyunsaturated fatty acids are then oxidized by either lipoxygenase or cyclooxygenase to form hydroperoxides or endoperoxides, respectively.
- 31. POWER RANGERNOTES FOOD CHEMISTRY 31 • Then these compounds are hydrolysed to yield a variety of breakdown products, which are responsible for the characteristic flavours of natural products. Pro-Oxidants • Transition metals, particularly those possessing two or more valency states and a suitable oxidation reduction potential between them are effective pro- oxidants. • e.g.copper, iron, manganese, cobalt and nickel • If present even at very low concentrations(0.1ppm) can decrease the induction period and increase the rate of oxidation. • Trace metals are naturally occurring in all food tissues and all fluids of biological origin (eggs, milk and fruit juices) and they are present in both free and bound forms. • Heme compounds are also important pro-oxidants. Antioxidant • An antioxidant is a molecule that can delay onset, or slow the rate of oxidation of oxidisable material. Oxidation reactions can produce free radical. In turn, these radicals can start chain reactions. • Antioxidants terminate these chain reactions by removing free radical intermediates, and inhibit other oxidation reactions by acting as hydrogen donors or free radical acceptors. • ROO. + AH → ROOH + A. • Antioxidants are found in varying amounts in foods such as vegetables, fruits, grain cereals, eggs, meat, legumes and nuts. • Antioxidants are widely used as preservatives in food and as ingredients in dietary supplements. • . Natural antioxidants: Ascorbic acid and tocopherols, • Synthetic antioxidants: Propyl gallate, isoamyl gallate, tertiary butylhydroquinone (TBHQ),butylated hydroxy anisole (BHA) and butylated hydroxy toluene (BHT). • Used as help guard against food deterioration • Antioxidants can be directly added to vegetable oils or to melted animal fats after they are rendered.
- 32. POWER RANGERNOTES FOOD CHEMISTRY 32 • Food products can also be dipped in or sprayed with solutions of antioxidants Digestion and absorption of lipids Introduction Foods are enzymatically digested to prepare them for absorption. During digestion in the gastrointestinal tract of mammals, the three major nutrients (carbohydrates, lipids, and proteins) undergo enzymatic hydrolysis into their building block components. This is necessary for their absorption, since the cells lining the intestine are able to absorb them into the bloodstream only as relatively small molecules. Lipids must be hydrolyzed into fatty acids and glycerol. Digestion The digestion of triglycerides begins in the small intestine. In this region the zymogen, prolipase is secreted by the pancreas (Fig.1). There it is converted into lipase, which in the presence of bile salts and a special protein called colipase, binds to droplets of triglycerides and catalyzes the hydrolytic removal of one or both of the outer fatty acid residues. Monoglycerides remain unhydrolyzed. The fatty acids and the uncleaved gycerides are emulsified into fine droplet by peristalsis, the churning action of the intestine, aided by the detergent effect of the bile salts and the monoglycerides, which are amphipathic molecules. Phospholipids are split by phospholipases to the acyl chains, glycerol and choline. Cholesterol esters are converted to cholesterol and free fatty acids. Absorption The fatty acids, glycerol and monogycerides, in these droplets are absorbed by intestinal cells, where they are largely reassembled into triglycerides. The free fatty acids are activated by thiokinase in the presence of coenzyme A and ATP for the resynthesis of triglyceride. Some free glycerol passes directly to the lymp vessel. The others will be activated by glycerokinase in the presence of ATP to form glycerol 3 phosphate and combine with acyl CoA to form triglycerides. All the long chain fatty acids present are reincorporated into the triglycerides. The triglycerides do not pass into the blood capillaries but into the small lymph vessels in the villi. The choline from phospholipids may be absorbed and send to liver via lymph vessels. Cholesterol is absorbed into the lymphatic vessels and converted into cholesterol esters and transported. Chylomicrons: The lymph draining the small intestine, called chyle, has a milky appearance after a fat-rich meal, due to the suspended chylomicrons, droplets of highly emulsified triglycerides, about 1µm in diameter. Chylomicrons contain
- 33. POWER RANGERNOTES FOOD CHEMISTRY 33 triglycerides, free and esterified cholesterol; have a hydrophilic coat of phospholipids and a special protein, which function to keep the chylomicrons suspended. The chylomicrons pass from the thoracic duct into the subclavian vein and then to liver. Emulsification: The emulsification and digestion of lipids in the small intestine is facilitated by the bile salts. The major human bile salts are sodium glycocholate and sodium taurocholate, derivative of cholic acid, the most abundant of four major human bile acids. The bile salts are powerful emulsifying agents secreted by the liver into the bile, which empties into the upper portion of the small intestine. After the fatty acids and monoglycerides of the emulsified fat droplets have been absorbed in the lower small intestine, the bile salts aiding this process are also reabsorbed. They return to the liver, to be used over again. Metabolism Metabolism of Triglycerides Triglycerides are first converted to fatty acids and glycerol mostly in adipose tissue. The fatty acids are released into the plasma where they combine with serum albumin. Long chain fatty acids are oxidized in liver, heart, kidney, muscle, lung, brain and adipose tissue. Glycerol is utilized by liver, kidney, intestine and lactating mammary gland where the activating enzyme glycerokinase is present. b) Metabolism of fatty acids The fatty acids components of the lipids entering the liver also have several different pathways 1. Oxidation to CO2 with ATP production: Free fatty acids may be activated and oxidized to yield acetyl-CoA and ATP. The acetyl-CoA is oxidized via the citric acid cycle to yield ATP by oxidative phosphorylation. Fatty acids are the major oxidative fuel in the liver. 2. Synthesis of fatty acids: There are three types of fatty acid synthesis. (1) Elongation of existing short chain fatty acid in the mitochondria (2) Microsomal system of chain elongation and (3) The cytoplasmic synthesis of fatty acid from acetyl CoA. 3. Biosynthesis of cholesterol: Some of the acetyl-CoA derived from fatty acids (and from glucose) will be used as the major precursor for the biosynthesis of cholesterol, which in turn is the precursor of the bile acids and bile salts, which are essential for the digestion and absorption of lipids. 4. Biosynthesis of lipids of plasma lipoproteins( Triglyceride and phospholipids): Fatty acids are also used as precursors for the synthesis of the lipid portion (triglycerides and phospholipids) of the plasma lipoproteins, which carry lipids to adipose or fat tissue for storage as triglycerides. 5. Fomation of ketone bodies: Excess acetyl-CoA released on oxidation of fatty acids and not required by the liver is converted into the ketone bodies, acetoactate and D-β-hydroxy butyrate, which are circulated via the blood to peripheral tissues, to be used as fuel for the citric acid cycle. The ketone bodies may be regarded as a transport form of acetyl groups. They can supply significant fraction of the energy to some peripheral tissues, up to one-third in the case of the heart
- 34. POWER RANGERNOTES FOOD CHEMISTRY 34 Metabolism of fat • The lipids of metabolic significance include synthesis and degradation of • triglycerides • phospholipids • steroids together with long chain fatty acids • glycerol and • ketone bodies • Oxidation of triglycerides takes place in the adipose tissue. • The complete degradation of fatty acid in the body leads to the oxidation to CO2 and water Metabolism of fatty acids The fatty acids components of the lipids entering the liver also have several different pathways 1. Oxidation to CO2 with ATP production 2. Biosynthesis of cholesterol 3. Biosynthesis of lipids of plasma lipoproteins (Triglyceride and phospholipids) 4. Formation of free fatty acids 5. Formation of ketone bodies Metabolism of Triglycerides • Triglycerides are first converted to fatty acids and glycerol mostly in adipose tissue.
- 35. POWER RANGERNOTES FOOD CHEMISTRY 35 • The fatty acids are released into the plasma where they combine with serum albumin. • Long chain fatty acids are oxidized in liver, heart, kidney, muscle, lung, brain and adipose tissue. • Glycerol is utilized by liver, kidney, intestine and lactating mammary gland where the activating enzyme glycerokinase is present. 1. Oxidation of fatty acids to CO2 with ATP production • Fatty acids are oxidized by β, α, and ω oxidation. β- Oxidation is the most important pathway for the production of energy. • The term β -oxidation means the oxidation takes place in the β -carbon in the fatty acid with the removal of 2 carbon atoms at a time from the carboxyl end of the molecule. • The saturated fatty acids containing even number and odd number of carbon atoms and the unsaturated fatty acids are oxidized by β -oxidation. (a) β-Oxidation of saturated fatty acids • Saturated fatty acids are oxidized to acetyl-CoA by β oxidation. • It takes place in mitochondria. • Five steps are involved and each step involves acy1-CoA derivatives catalyzed by separate enzymes, utilizes NAD+ and FAD as coenzymes, and generates ATP. • Fatty acid oxidation is an aerobic process, requiring the presence of oxygen. Step 1 Activation of Fatty Acids • Long chain fatty acids are first converted to an ‘active fatty acid’ or acyl CoA in the cytosol • But activation of lower fatty acids occurs within the mitochondria. • Thiokinase is found both inside and outside the mitochondria. • Thiokinase Fatty acid+ATP+coenzyme A +Mg2+→Acyl CoA +AMP • The presence of inorganic pyrophosphatase ensures that activation goes to completion by facilitating the loss of the additional high-energy phosphate associated with pyrophosphate.
- 36. POWER RANGERNOTES FOOD CHEMISTRY 36 • Two high energy phosphates are expended during the activation of each fatty acid molecule. • (i)Transport of smallerfatty acids • Small fatty acids are able to penetrate the inner membrane off mitochondria and become oxidized within the mitochondria. • (ii) Transport of long-chainfatty acids • Long-chain fatty acid penetrate the inner mitochondrial membrane only as carnitine derivatives. • Carnitine acyl transferase I, -in outer mitochondrial membrane, converts long- chain acyl CoA to acyl carnitine, -penetrate the inner membrane of mitochondria • Carnitine-acyl carnitine translocase -in mitochondria, catalyses the transfer the acylcarnitine into inner membrane. • Carnitine acyl transferase II- in the inner mitochondrial membrane, converts acyl carnitine to long-chain acyl CoA and carnitine. • Acyl CoA then undergoes further reactions of β-oxidation Step 2 Dehydrogenation of Aceyl CoA Aceyl CoA dehydrogenase Acyl CoA + NAD+ ↔ α-β unsaturated acyl CoA + NADH + H+ NADH + H+ is reoxidised via electron transport chain. Step 3 Conversion of α-β unsaturated acyl CoA to β hydroxyl acyl CoA Enoyl-CoA hydratase. α-β unsaturated acyl CoA + H2O↔ β hydroxyl acyl CoA Step4 Dehydrogenation at the β-carbon of β-hydroxyacyl CoA β-hydroxyacyl-CoA dehydrogenase β hydroxyl acyl CoA +NAD+ ↔β-ketoacyl-CoA+ NADH+H + The NADH+H+ formed is reoxidised via electron transport chain.
- 37. POWER RANGERNOTES FOOD CHEMISTRY 37 Step5. Cleavage by thiolase Thiolase β-ketoacyl-CoA ↔ Acetyl-CoA + acyl-CoA The products of this reaction are acetyl-CoA and an acyl-CoA derivative containing two carbons less than the original acyl-CoA molecule that underwent this oxidation. The acyl-CoA formed in the cleavage reaction renters the oxidative pathway at reaction 1. A long chain fatty acid may be degraded completely to acetyl-CoA (C2 units). In the case of palmitic acid the reactions are repeated 7 times and 8 molecules of acetyl CoA are formed. Since acetyl-CoA can be oxidized to CO2 and water via the citric acid cycle, the complete oxidation of fatty acids is achieved Production of ATP Synthesis of high energy phosphates (ATP) for Electron transport chain reoxidation of FADH2 and NADH of formation of 7 acetyl-CoA molecules for β- oxidation of palmitate : five. No.of ATP derived from β-Oxidation Since 8 molecules of acetyl CoA are formed : 7x5 = 35 No. of ATP formed on oxidation of 8 acetyl-CoA molecules (via citric acid cycle) : 8x12 = 96 -------- Total : 131 ATP utilized for initial activation of the fatty acid: -2 ------- Net total yield : 129 Calorific value per mole of palmitic acid: • The calorific value is 129x7.6=980 K.cal/mole.
- 38. POWER RANGERNOTES FOOD CHEMISTRY 38 • The calorific value per mole of combustion of palmitic acid is 2340 K.cal/mole. • The process captures as high-energy phosphate in the order of 41% of the total energy of combustion of the fatty acid. (β)Oxidation of a fatty acid with an odd number of carbon atoms Fatty acids with an odd number of carbon atoms are oxidized by the pathway of β - oxidation, producing acetyl-CoA until a three- carbon (propionyl-CoA) residue remains. This compound is converted to succinyl-CoA, a constituent of the citric acid cycle and metabolized. Propionyl-CoA carboxylase Propionyl CoA + CO2 + H2O → D-methylmalonyl-CoA D-Methylmalonyl-CoA is converted to its steroisomer, L- methylmalonyl-CoA, by methylmalonyl-coA racemase before its final isomerization to succinyl-CoA by the enzyme methylmalonyl-CoA isomerase. Methylmalonyl-CoA racemase D-Methylmalonyl-CoA ↔ L- methylmalonyl-CoA Methylmalonyl-CoA isomerase L- methylmalonyl-CoA ↔ Succinyl-CoA Thus the propiony fatty acid l residue from an odd-chain fatty acid is the only part of a b) Oxidation of unsaturted fatty acids Oxidation of unsaturated fatty acids occurs by a modified beta- oxidation pathway. 1. Initial reaction The CoA ester of these acids are degraded by the enzymes normally responsible for β - oxidation until either a Δ3 -cis- acyl-CoA compound or Δ4 -Cis-acyl-CoA compound is formed, depending upon the position of the double bonds. 2. Reaction of Isomerase
- 39. POWER RANGERNOTES FOOD CHEMISTRY 39 The former compound is isomerized (Δ3 cis-Δ2 Acyl CoA isomerase) to the corresponding Δ2 -trans-CoA stage of β - oxidation for subsequent hydration and oxidation. (One cycle of Beta Oxidation) 3. Conversion of α-β unsaturated acyl CoA to β hydroxyl acyl CoA Enoyl-CoA hydratase. α-β unsaturated acyl CoA + H2O↔ β hydroxyl acyl CoA 4.Dehydrogenation at the β-carbon of β-hydroxyacyl CoA β-hydroxyacyl-CoA dehydrogenase β hydroxyl acyl CoA +NAD+ ↔β-ketoacyl-CoA+ NADH+H + 5. Action of thiolase 6. Conversion of Δ4 -cis-acy1-CoA to Δ2 -trans enoy1-CoA Any Δ4 -cis-acy1-CoA remaining, as in the case of linoleic acid, is converted to Δ2 -trans enoy1-CoA by an NADP dependent enzyme, Δ2 - trans - Δ3-cis dienoy1-CoA reductase. 7. Action of Acyl-CoA dehydrogenase Δ-cis (or trans) Δ2 enoy1-CoA isomerase will attack the trans double bond to produce Δ2 - trans enoy1-CoA, This compound is further metobolised via β - oxidation DIGESTION AND ABSORPTION OF LIPIDS INTRODUCTION • Foods are enzymatically digested to prepare them for absorption. • During digestion in the gastrointestinal tract of mammals, the three major nutrients (carbohydrates, lipids, and proteins) undergo enzymatic hydrolysis into their building block components. • This is necessary for their absorption, since the cells lining the intestine are able to absorb them into the bloodstream only as relatively small molecules. • Lipids must be hydrolyzed into to fatty acids and glycerol.
- 40. POWER RANGERNOTES FOOD CHEMISTRY 40 Digestion and absorption of fat • The digestion of triglycerides beings in the small intestine, In this region the zymogen, prolipase is secreted by the pancreas (Fig.4.1). • There it is converted into active lipase, which in the presence of bile salts and a special protein called colipase, binds to droplets of triglycerides and catalyzes the hydrolytic removal of one or both of the outer fatty acid residues. • Monoglycerides remain unhydrolyzed. • The fatty acids and the uncleaved gycerides are emulsified into fine droplet by peristalsis, the churning action of the intestine, acided by the detergent effect of the bile salts and the monoglycerides, which are amphipathic molecules. • Phospholipids are split by phospholipases to the acyl chains, glycerol and choline. • Cholesterol esters are converted to cholesterol and free fatty acids Absorption • The fatty acids, glycerol and monogycerides, in these droplets are absorbed by intestinal cells, where they are largely reassembled into triglycerides. • The free fatty acids are activated by thiokinase in the presence of coenzyme A and ATP for the resynthesis of triglyceride. • Some free glycerol passes directly to the lymp vessel. • The others will be activated by glycerokinase in the presence of ATP to form glycerol 3 phosphate and combine with acyl CoA to form triglycerides. • All the long chain fatty acids present are reincorporated into the triglycerides. • The triglycerides do not pass into the blood capillaries but into the small lymph vessels in the villi.
- 41. POWER RANGERNOTES FOOD CHEMISTRY 41 • The cholin from phospholipids may be absorbed and send to liver via lymph vessels. • Cholesterol is absorbed into the lymphatic vessels and converted into cholesterol esters and transported. Chylomicrons The lymph draining the small intestine, called chyle. It has a milky appearance after a fat-rich meal, due to the suspended chylomicrons, droplets of highly emulsified triglycerides, about 1µm in diameter. Chylomicrons contain triglycerides, free and esterified cholesterol; have a hydrophilic coat of phospholipids and a special protein, which function to keep the chylomicrons suspended. The chylomicrons pass from the thoracic duct into the subkavian vein and then to liver. Emulsification The emulsification and digestion of lipids in the small intestine is facilitated by the bile salts. The major human bile salts are sodium glycocholate and sodium taurocholate, derivative of cholic acid, the most abundant of four major human bile acids. The bile salts are powerful emulsifying agents secreted by the liver into the bile, which empties into the upper portion of the small intestine. After the fatty acids and monoglycerides of the emulsified fat droplets have been absorbed in the lower small intestine, the bile salts aiding this process are also reabsorbed. They return to the liver, to be used over again. METABOLISM OF LIPIDS a) Metabolism of Triglycerides b) Metabolism of fatty acids
- 42. POWER RANGERNOTES FOOD CHEMISTRY 42 a) Metabolism of Triglycerides • Triglycerides are first converted to fatty acids and glycerol mostly in adipose tissue. • The fatty acids are released into the plasma where they combine with serum albumin. • Long chain fatty acids are oxidized in liver, heart, kidney, muscle, lung, brain and adipose tissue. • Glycerol is utilized by liver, kidney, intestine and lactating mammary gland where the activating enzyme glycero kinase is present. b) Metabolism of fatty acids The fatty acids components of the lipids entering the liver also have several different pathways. 1. Oxidation to CO2 with ATP production Free fatty acids may be activated and oxidized to yield acetyl-CoA and ATP via glycolysis. The acetyl-CoA is oxidized via the citric acid cycle to yield ATP by oxidative phosphorylation. Fatty acids are the major oxidative fuel in the liver. 2. Biosynthesis of cholesterol and bile salts • Some of the acetyl-CoA derived from fatty acids (and from glucose) will be used as the major precursor for the biosynthesis of cholesterol, • Cholesterol is the precursor of the bile acids and bile salts, which are essential for the digestion and absorption of lipids. 3.Biosynthesis of plasma lipoproteins Fatty acids are also used an precursors for the synthesis of the lipid portion of the plasma lipoproteins. Lipoproteins carry lipids to adipose or fat tissue for storage as trigycerides. . 4.Formation of plasma free fatty acids
- 43. POWER RANGERNOTES FOOD CHEMISTRY 43 Free fatty acids become bound to serum albumin and are carried via the blood to the heart and skeletal muscles, which absorb and oxidize free fatty acids as major fuel. 5. Formation of ketone bodies Excess acetyl-CoA released on oxidation of fatty acids and not required by the liver is converted into the ketone bodies, acetoactate and D-β-hydroxy butyrate, which are circulated via the blood to peripheral tissues, to be used as fuel for the citric acid cycle. The ketone bodies may be regarded as a transport form of acetyl groups. They can supply significant fraction of the energy of some peripheral tissues, up to one-third in the case of the heart. Metabolism of Fatty acids CARBOHYDRATES Introduction Nature commonly utilizes carbohydrates as source of energy, structure-forming material, water-maintaining hydrocolloids and even sex attractants. Amino acids synthesize in the concentrated space and polymerize into proteins on already-available polysaccharide matrices.
- 44. POWER RANGERNOTES FOOD CHEMISTRY 44 Carbohydrates are organic compounds containing carbon, hydrogen and oxygen with the general formula Cn(H2O)n. They may be simple or complex molecules. Important food carbohydrates include simple sugars, dextrins, starches, celluloses, hemicelluloses, pectin, and gums. They are an important source of energy or fiber in the diet and they are important constituents of food because of their functional properties. They are used as sweeteners, thickeners, stabilizers, gelling agents, and fat replacers. The simplest carbohydrates are called monosaccharides or sugars and they have the general formulae CnH2nOn. The most common ones contain six carbon atoms. Disaccharide contains two sugar units, trisaccharides contain three, oligosaccharides contain several units, and polysaccharides are complex polymers containing as many as several thousand units of monosaccharides linked by means of glycosidic bonds. Monosaccharides • Monosaccharides are simple carbohydrates containing between three and eight carbon atoms, but only those with five and six carbon atoms are common. • Most important ones are glucose and fructose with general formula C6H12O6. • Glucose is an aldose as it contains an aldehyde group. • Fructose is a ketose as it contains a keto group. Monosaccharide and their natural derivatives Pentoses L-Arabinose D-xylose Plant gums, hemicellulose, saponins, protopectin Accompanies L- arabinose Alcoholic fermentation, furan-2, aldehyde production Reduction to xylitol
- 45. POWER RANGERNOTES FOOD CHEMISTRY 45 sucrose substitute; alcoholic fermentation; production of furan-2 aldehyde Hexoses D-Glucose D-Fructose D-Galactose L-Fucose D-Mannose L-Rhamnose Plants and animals, honey, inverted sugar, saponins Fruit, traces in plant honey, Constituent of milk, dairy products (Milk sugar) algae, plant mucus and gums Oligo-and polysaccharides, plant mucus and gums, saponins, glycosides Algae, plant mucus. Plant mucus and gums, pectins saponins, glycosides Alcoholic fermentation; sweetener ; energy pharmacopeial material; nutrient; food preservative Preparation of dairy products Preparation of mannitol, which is used as an alternative sweetener in food products Hexuloses D-Fructose D- Glucosylamine L-Sorbose Fruits, honey, inverted sugar maple syrup Chitin, Chitosan Rowan berries Noncavity-causing sweetener; sweetener for diabetics; food humidifier and preservative Pharmaceutical aid; antiarthritic drugs; ion exchanger Synthesis of ascorbic acid Disaccharides • Disaccharide contains two monosaccharides linked together by glycosidic bond. Sucrose or the table sugar is the most common disaccharide.
- 46. POWER RANGERNOTES FOOD CHEMISTRY 46 • It contains glucose and fructose linked by α-1, 2-glycosidic bond. • Maltose contains two glucose units linked by α1-4 glycosidic bond. • Lactose known as milk sugar contains one glucose and one galactose molecules. • Maltose is the building block of starch, which contains Disaccharides Lactose Maltose Sucrose Mammalian 'milk Starch, sugar beet, honey Sprouted grain, hydrolysis of starch Sugar beet, sugar cane , maple syrup Dairy product taste improver; fermenting component of milk Food fermentation; Common sweetener; caramel production; food preservation Oligosaccharides • Oligosaccharides contain 3-10 monosaccharide units linked together by glycosidic bonds. • Common ones include raffinose and stachyose. • Raffinose is a trisaccharide with galactose, glucose and fructose. Stachyose contains glucose, fructose and two galactose units. • Both occur in legumes and dry beans and peas. • They are not hydrolyzed or digested by human digestive system and become food for bacteria in the large intestine. Polysaccharides • Polysaccharides contain more than 10 monosaccharide units linked together by glycosidic bond. • The most important polysaccharides are starches, pectins and gums. • All are complex polymers with different properties, which depend on the mono saccharides that make up the structure the linkage by which they are linked and the degree of branching of the molecules. Occurrence
- 47. POWER RANGERNOTES FOOD CHEMISTRY 47 • All organism cells, including those of animals, contain components of carbohydrates in their membranes. • Frequently, carbohydrates exist in naturally derivatives forms, including aminated forms, as in chitin and chitosan; esterified; alkylated as in glycosides; oxidized; reduces; or linked to proteins, lipids, and other structures such as glycoproteins. • Lower monosaccharides, such as aldotrioses and aldo-and ketotetroses, do not exist naturally in a free state. • Glyceraldehyde in phosphorylated form is the product of alcoholic fermentation and glycolic sequence. • Erythrose, an aldotetrose, and erythrulose, a ketotetrose, also appear in phosphorylated from in the pentose cycle of glucose, while ketopentose- ribulose can be found as its phosphate ester. • Several common and uncommon carbohydrates (erlose, turanose, trehalose, isomaltose, melecitose) have been found in honey. In nature, various carbohydrates derivatives are also found. • Among them are so-called sugar alcohols (auditors). • They are the natural products of the reduction of monosaccharide. • Algal gums and mucilage’s constitute an abundant group of polysaccharides in plants. FIBER IN FOOD AND ITS ROLE 1. Introduction • Non-starch polysaccharide is the main components of dietary fiber. • Pectin, gum, mucilage, cellulose, hemicelluloses and lignin. • Dietary fiber comes from the portion of plants that is not digested by enzymes in the intestinal tract. Part of it, however, may be metabolized by bacteria in the lower gut. • Different types of plants- have varying amounts and kinds of fiber. . Soluble Fiber • Pectin and gum are water-soluble fibers found inside plant cells. • They slow the passage of food through the intestines but do nothing to increase fecal bulk.
- 48. POWER RANGERNOTES FOOD CHEMISTRY 48 • Beans, oat bran, fruit and vegetables contain soluble fiber Water insoluble fibers • Present in cell walls. • Cellulose, hemicelluloses and lignin. • Such fibers increase fecal bulk and speed up the passage of food through the digestive tract. • Wheat bran and whole grains - the most insoluble fiber- vegetables and beans - are good sources 2. Benefits of Fiber i) Prevent constipation Insoluble fiber binds water, making stools softer and bulkier. Therefore, fiber especially that found in whole grain products is helpful in the treatment and prevention of constipation, hemorrhoids and diverticulosis. Diverticula are pouches of the intestinal wall that can become inflamed and painful. In the past, a low-fiber diet was prescribed for this condition. A high-fiber diet gives better results once the inflammation has subsided. ii) Lower cholesterol levels • Low blood cholesterol levels (below 200 mg/dl.) have been associated with a reduced risk of coronary heart disease. • The body eliminates cholesterol through the excretion of bile acids. Water- soluble fiber binds bile acids, and hence a high-fiber diet may result in an increased excretion of cholesterol. • Some types of fiber appear to have a greater effect than others. • The fiber found in rolled oats is more effective in lowering blood cholesterol levels than the fiber found in wheat. • Pectin has a similar effect in that it, too, can lower the amount of cholesterol in the blood. iii) Reduce the risk of some cancers Dietary fiber may help reduce the risk of some cancers, especially colon cancer. This idea is based on information that insoluble fiber increases the rate at which wastes are removed from the body.
- 49. POWER RANGERNOTES FOOD CHEMISTRY 49 This means the body may have less exposure to toxic substances produced during digestion. A diet high in animal fat and protein also may play a role in the development of colon cancer. iv) Useful for losing weight High-fiber diets may be useful for people who wish to lose weight. Fiber itself has no calories, yet provides a "full" feeling because of its water- absorbing ability. For example, an apple is more filling than a half cup of apple juice that contains about the same calories. Foods high in fiber often require more chewing, so a person is unable to eat a large number of calories in a short amount of time. 3. Sources of Fiber • Dietary fiber is found only in plant foods: fruits, vegetables, nuts and grains. Meat, milk and eggs do not contain fiber. • The form of food may or may not affect its fiber content. • Canned and frozen fruits and vegetables contain just as much fiber as raw ones. • Other types of processing, though, may reduce fiber content. • Drying and crushing, for example, destroy the water-holding qualities of fiber. • The removal of seeds, peels or hulls also reduces fiber content. • Whole tomatoes have more fiber than peeled tomatoes, which have more than tomato juice. • Likewise, whole wheat bread contains more fiber than white bread. 4. RDA • Women 25 grams per day, for women younger than 50 21 grams per day, for women older than 50
- 50. POWER RANGERNOTES FOOD CHEMISTRY 50 • Men 38 grams per day, for men younger than 50 30 grams per day, for men older than 50 5. Adverse effect • Although fiber is important, it is just one part of a properly balanced diet. • Too much fiber may reduce the amount of calcium, iron, zinc, copper and magnesium that is absorbed from foods. • Deficiencies of these nutrients could result if the amount of fiber in the diet is excessive, especially in young children. • Fiber supplements are sold in a variety of forms from bran tablets to purified cellulose. • Many laxatives sold as stool softeners actually are fiber supplements. • Fiber's role in the diet is still being investigated. • Various types of fiber have different roles in the body. • For these reasons fiber supplements should be avoided. • Eating a variety of fiber-rich foods is the best way to receive the maximum benefits from each type of fiber present in foods, and obtain necessary nutrients. BROWNING REACTIONS 1. Introduction Browning is a common colour change seen in food during pre-preparation, processing or storage of food. It occurs in varying degrees in some foods. The colour produced range from cream or pale yellow to dark brown or black. Browning reactions observed in food may be classified as enzymatic browning or nonenzymatic browning. a) Enzymatic Browning
- 51. POWER RANGERNOTES FOOD CHEMISTRY 51 • Fruits such as apples, pears, peaches, apricots, and bananas, and vegetables such as potatoes quickly turn brown when their tissue is exposed to oxygen. • Such oxygen exposure occurs when the food is sliced or bitten into or when it has sustained bruises, cuts or other injury to the peel. • This “browning reaction” is related to the work of an enzyme called phenolase (or polyphenoloxidase), a conjugated enzyme in which copper is present. Phenolase • Phenolase is classified as an oxidoreductase. • The substrates for phenolase are phenolic compounds present in the tissues of the fruits and vegetables. Phenolase hydroxylates monophenols to 0-diphenol and oxidizes 0-diphenols to 0-quinones. • The 0-quinones then enter into a number of other reactions, which produce the “undesirable” brown discolorations. • Quinone formation is enzyme and oxygen-dependent. • Once the quinones have formed, the subsequent reactions occur spontaneously and no longer depend on the presence of phenolase or oxygen. Prevention Enzymatic browning can be prevented or slowed in several ways. Immersing the “injured” food (for example, apple slices) in cold water slows the browning process. The optimum temperature for enzymes to act is 43ºC(109ºF).The lower temperature decreases enzyme activity, and the water limits the enzyme’s access to oxygen. Refrigeration slows enzyme activity even more, and boiling temperatures destroy (denature) the enzyme. A long-used method for preventing browning involves lowering of pH to 2.5-2.7 by the addition of acids such as ascorbic acid, malic or citric acid Phenolase works very slowly in the acidic environment created by the added acids. In addition, the vitamin C (ascorbic acid) present in lemon juice functions as an antioxidant.
- 52. POWER RANGERNOTES FOOD CHEMISTRY 52 It is more easily oxidized than the phenolic-derived compounds, and its oxidation products are colorless. b) Non Enxymatic Browning 1) Maillard reaction: • The non enzymatic browning or Maillard reaction is a chemical reaction between an amino acid and a reducing sugat, usually requiring heat. • When aldoses and ketoses are heated with amines, a variety of reactions ensue, producing numerous compounds some of which are flavours, aromas and dark coloured polymeric material. • They may be produced slowly during storage and much more rapidly at the high temperature encountered during frying roasting or backing. • The reducing sugar reacts with the amine to form a Schiff base (an imines) which may cyclate to form glucosamine. • In the case of glucose the Schiff base undergo a reaction called Amadori rearrangement to give 1-amino-1-deoxy-D-fructose or Amadori compound. • The Amadori compounds are early intermediates in the browning reaction sequence. • Amadori compounds undergo transformation via different pathways starting with four different intermediates formed from them. • The result is a complex mixture of intermediates and products. The Maillard reaction occurs in three main steps: • 1. Initial step- formation N glycoside: The carbonyl group of the sugar reacts with the amino group of the amino acid, producing N-substituted glycosylamine and water • 2. After formation of N glycoside the immonium ion is formed and then isomerizes, this reaction is called Amadori rearrangement and forms a compound called ketosamine: • 3. The ketosamine products then either dehydrates into reductones and dehydro reductones, which are caramel, or products -short chain hydrolytic fission products such as diacetyl, acetol or pyruvaldehyde which then undergo the Strecker degradation and produce short-chain hydrolytic fission products and brown nitrogenous polymers and melanoidins
- 53. POWER RANGERNOTES FOOD CHEMISTRY 53 • Important intermediates are formed by rearrangements and eliminations are 1-, 3- and 4-deoxydicarbonyl compounds called 1-, 3-, and 4-deoxyosones. They finally form 5- hydroxy methyl furfural • In the process, hundreds of different flavor compounds are created. These compounds in turn break down to form yet more new flavor compounds, and so on. Each type of food has a very distinctive set of flavor compounds that are formed during the Maillard reaction. It is these same compounds have been used over the years to create artificial flavors. Food products with Maillard reactions • The Maillard reaction is responsible for many colors and flavors in foods such as bread, biscuit, malted barley as in malt whiskey or beer, roasted meat, dried or condensed milk, roasted coffee etc 6-Acetyl-2,3,4,5-tetrahydropyridine is responsible for the biscuit or cracker-like flavor present in baked goods like bread and popcorn. • The structurally related compound 2-acetyl-1 pyrrpoline has a similar smell and occurs also naturally without heating and gives varieties of cooked rice their typical smell. • Maillard reaction may result in a reduction in nutritional properties and the formation of potentially toxic and mutagenic compounds. In a food system, the reactants are mostly amino acids (free forms or peptide-bound) and reducing sugars. • Since up to 50% of the food groups have been processed before consumption, some of the amino acids and reducing sugars is lost during processing. • Maillard reactions affect protein bioavailability by derivatizing protein-bound, dietary limiting amino acids such as lysine, arginine, and histidine. • Maillard reaction products also exhibit antinutritive effects by mechanism involving complex formation with micronutrients, destruction of vitamins, and by acting as inhibitors of digestive enzymes • High temperature, low moisture levels and alkaline conditions promote the Maillard reaction. • The rate of Maillard reactions increases as the water activity increases, reaching a maximum at water activities in the range of 0.6 to 0.7. • However, as the Maillard reaction produces water, further increases in water activity may inhibit Maillard reactions. • Pentose sugars react more than hexoses, which react more than disaccharide. • Different aminoacids produce different amounts of browning
- 54. POWER RANGERNOTES FOOD CHEMISTRY 54 2) Browning reactions which occur in meat • The browning reactions which occur when meat is roasted or seared have often been referred to as Maillard reaction browning. • However, lean meat contains very few, if any, reducing sugars. • Furthermore, red meat undergoes more extensive browning than does white meat. • The browning reactions in lean meat are most likely due to the breakdown of the tetrapyrrole rings of the muscle protein, myoglobin. • Thus, the browning of meat is technically not a Maillard browning since it does not involve the reaction with a reducing sugar 3) Caramelization • Caramelization is a browning reaction formed by heating carbohydrates like sucrose or reducing sugars. • Reactions are facilitated by small quantity of acids, base and certain salts. • Caramelization is an entirely different process from Maillard browning, though the results of the two processes are sometimes similar to the naked eye (and taste buds). • The final product caramel contains a complex mixture of polymeric compound, formed from unsaturated cyclic compounds. • Flavour and aroma compounds are also formed. • Heating causes the dehydration of sugar molecule with introduction of double bonds or formation of anhydro rings. Intermediates such as 3-deoxy osones and furans are formed. • The unsaturated rings may condense to form useful, conjugated double-bond containing, brown coloured polymers. • Catalysts increase the reaction rate and are used to direct the reaction to specify types of caramel colour, solubility and acidities. • To make caramel a carbohydrate is heated alone or in the presence of acid, a base or salt. • The carbohydrates most often used are sucrose, but fructose, glucose, invert sugar, malt syrups and molasses may also be used. • Acid used are food grade sulfuric, sulfurous, phosphoric, acetic and citric acids.
- 55. POWER RANGERNOTES FOOD CHEMISTRY 55 • Bases that may be used are ammonium, sodium, potassium and calcium hydroxides. • Salts that may be used are ammonium, sodium, potassium carbonates, bicarbonates, phosphates, sulphates or bisulphates. Classes of caramel Class I caramel :Prepared by heating a plain carbohydrate Class II caramel :Prepared by heating a carbohydrate in the presence of a sulphite Class III caramel :Prepared by heating a carbohydrate in the presence of a source of ammonium ion. Class IV caramel :Prepared by heating a carbohydrate in the presence of a both sulphite and ammonium ions • Caramelization may sometimes cause browning in the same foods in which the Maillard reaction occurs, but the two processes are distinct. • They both are promoted by heating, but the Maillard reaction involves amino acids, as discussed above, while caramelization is simply the pyrolysis of certain sugars. DIGESTION AND ABSORPTION OF CARBOHYDRATES INTRODUCTION The most abundant carbohydrates ingested by human beings are they polysaccharides, starch and cellulose, furnished by plant foods and glycogen, provided by foods of animal origin. 2. Digestion- Starch • Starch and glycogen are completely hydrolyzed by enzyme action in the gastrointestinal tract to yield free D-glucose. • This process begins in the mouth during chewing, through the action of amylase secreted by the salivary glands. • Salivary amylase hydrolyzes many of the α (14) glycosidic linkages of starch and glycogen Salivary amylase Starch and glycogen → a mixture of maltose, glucose and
- 56. POWER RANGERNOTES FOOD CHEMISTRY 56 oligosaccharides. The digestion of digestible polysaccharides to yield D-glucose is continued and completed in the small intestine 1.Pancreatic amylase -made by the pancreas and secreted via the pancreatic duct into the upper protein of the small intestine duodenum 2.Intestinal amylase secreted by small intestine continue and complete the digestion of starch Pancreatic amylase & intestinal amylase Starch → a mixture of maltose, glucose and oligosaccharides Disaccharides Disaccharides are hydrolyzed by enzymes located in the outer border of the epithelial cells lining the small intestine. sucrase or invertase Surcose → D-glucose and D-fructose , lactase Lactose → D-glucose and D-galactose maltase Maltose → two molecules of D-glucose. The liver stores the glucose as glycogen and releases glucose as and when needed to maintain blood glucose level. Cellulose • Cellulose cannot be enzymatically digested and used by most mammals for lack of enzymes capable of hydrolyzing the β(14) linkages between successive D-glucose residues of cellulose.
- 57. POWER RANGERNOTES FOOD CHEMISTRY 57 • Nevertheless undigested cellulose residues of plant foods provide bulk or fiber (also called “roughage”) in the diet and are desirable for proper motility of materials in the intestine. • Cellulose can be digested by ruminant animals, but only indirectly. The rumen bacteria hydrolyze cellulose to yield D-glucose, which they ferment to yield lactate, acetate, and propionate, absorbed into the blood. • Lactate and propionate are converted by the liver into glucose sugar in ruminants. Absorption • In the epithelial cells lining the small intestine, D-fructose, D-galactose and D- mannose are converted into D-glucose. • The resulting mixture of simple hexoses is absorbed into the epithelial cells lining the small intestine and brought via the blood to the liver. Metabolisms involving glucose • The absorbed free glucose is phosphorylated to glucose 6 phosphate by hexokinase using ATP. • The fructose, galactose and mannose absorbed are also converted into glucose 6 phosphate. • This compound is metabolized by five major metabolic path ways. i. Conversion into blood glucose • The absorbed free glucose is phosphorylated to glucose 6 phosphate by hexokinase using ATP. • The fructose, galactose and mannose absorbed are also converted into glucose 6 phosphate. • This compound is then metabolized by major metabolic path ways. • Glucose 6-phosphate is dephosphorylated by glucose 6-phosphate to yield free-D-glucose, which passes into the systemic blood to be transported to other tissues. Glucose 6 phosphatase • Glucose 6-phosphate → Glucose + Pi ii. Conversion into glycogen
- 58. POWER RANGERNOTES FOOD CHEMISTRY 58 Glucose 6-phosphate not immediately needed to form blood glucose ↓ Converted into liver glycogen by the sequential action of phosphoglucomutase and glycogen synthase. iii. Conversion into fatty acids and cholesterol Excess glucose 6-phosphate not used to make blood glucose or liver glycogen ↓ Degraded via glycolysis and pyruvate dehydrogenase into acetyl-CoA Acetyl-CoA → malonyl CoA -----→Fatty acids. ↓ triglycerides and phospholipids ↓ Transported to other tissues by plasma lipoproteins. Acetyl-CoA → to cholesterol by liver iv. Oxidative degradation to CO2 1. Glycolysis Glucose 6-phosphate to pyruvate 2. Decarboxylation Pyruvate to Acetyl-CoA, oxidized via the. 3. Citric acid cycle Acetyl-CoA oxidized to Co2 and H2O 4.Electron transport and oxidative phosphorylation yield energy in the form of ATP. Fatty acids are the major oxidative fuel for the citric acid cycle in the liver.
- 59. POWER RANGERNOTES FOOD CHEMISTRY 59 5. Degradation via the Pentose Phosphate Pathway Glucose 6- phosphate →the pentose phosphate pathway ↓ (1) Reducing power in the form of NADPH, needed in the reducing steps in the biosynthesis of fatty acids and cholesterol and (2) D-ribose 5- phosphate, a precursor in nucleotide biosynthesis. Through the action of various regulatory enzymes and through hormonal regulation, the liver directs the flow of glucose residues into these different pathways according to the prevailing supply and demand economy of the organism. Digestion of carbohydrates
- 60. POWER RANGERNOTES FOOD CHEMISTRY 60 GLUCONEOGENESIS • Gluconeogenesis is the synthesis of glucose from noncarbohydrate and then conversion to glycogen • The major substrates for gluconeogenesis are the glucogenic amino acids, lactate, glycerol, and propionate. • Liver and kidney are the major tissues involved, since they contain a full complement of the necessary enzymes. Importance • Gluconeogenesis meets the needs of the body for glucose when carbohydrate is not available in sufficient amounts from the diet. • A continual supply of glucose is necessary as a source of energy, especially for the nervous system and the erythrocytes. Failure of gluconeogensis is usually fatal. Below a critical blood glucose concentration, there is brain dysfunction, which can lead to coma and death. • Glucose is also required in adipose tissue as a source of glyceride-glycerol, and it probably plays a role in maintaining the level of intermediates of the citric acid cycle in many tissues. • Even under conditions where fat may be supplying most of the caloric requirement of the organism, there is always a certain basal requirement for glucose.
- 61. POWER RANGERNOTES FOOD CHEMISTRY 61 • Glucose is the only fuel that will supply energy to skeletal muscle under anaerobic conditions. • In addition, gluconeogenic mechanisms are used to clear the products of the metabolism of other tissues from the blood, e.g. lactate, produced by muscle and erythrocytes, and glycerols, which is continuously produced by adipose tissue. • Gluconeogenesis involves glycolysis, the citric acid cycle, plus some special reactions
- 62. POWER RANGERNOTES FOOD CHEMISTRY 62 The energy barriers obstruct a simple reversal of glycolysis between pyruvate and phosphoenolpyruvate, between furctose 1,6 bisphosphate and furctose 6-phosphate, between glucose 6-phosphate and glucose, and between glucose 1-phosphate and glycogen. These reactions are all nonequilibrium, releasing much free energy as heat and therefore physiologically irreversible. These reactions are circumvented by the following special reactions. A. Conversion of Pyruvate into Phosphoenolpyruvate: Pyruvate carboxylase, present in mitochondria, converts pyruvate to oxaloacetate in the presence of ATP, the B vitamin biotin, and CO2. The function of the biotin is to bind CO2 from bicarbonate onto the enzyme prior to the addition of the CO2 to pyruvate. A second enzyme, phosphoenolpyruate carboxykinase, catalyzes the conversion of oxaloacetate to phosphoenolpyruvate. High energy phosphate in the form of GTP or ITP is requried in this reaction, and CO2 is liberated. Thus, with the help of these two enzymes catalyzing endergonic transformations and lactate dehydrogenase, lactate can be converted to phosphoenolpyruvate, overcoming the energy barrier between pyruvate and phosphoenolpyruvate. B. Conversion of Fructose 1,6-Bisphosphate into Fructose 6- phosphate: The conversion of fructose1,6 bisphosphate to fructose 6-phosphate, necessary to achieve a reversal of glycolysis, is catalyzed by a specific enyzme, fructose-1,6 bisphosphatase. This enzyme is present in liver and kidney and in striated muscle. It is absent in heart muscle and smooth muscle. C. Conversion of glucose 6-phosphate into Glucose:
- 63. POWER RANGERNOTES FOOD CHEMISTRY 63 The conversion of glucose 6-phosphate to glucose is catalyzed by another specific phosphatase, glucose-6-phosphate.It’s presence allows a tissue to add glucose to the blood. D. Conversion of glucose 6-phosphate into Glycogen: The break down of glycogen to glucose 1-phosphate is carried out by phosphorylase. The synthesis of glycogen involves an entirely different pathway through the formation of uridine diphosphate glucose and the activity of glycogen synthase. These key enzymes allow reversal of glycolysis to play a major role in gluconeogenesis, the relationships between gluconeogenesis and the glycolytic pathway. After transamination or deamination, glucogenic amino acids form either pyruvate or members of the citric acid cycle. The reactions described above can account for the conversion of both glucogenic amino acids and lactate to glucose or glycogen. Thus, lactate forms pyruvate and enter the mitochondria before conversion to oxaloacetate and ultimate conversion to glucose. The source of pyruvate and oxaloacetate for gluconeogenesis is mainly amino acid catabolism. • Some amino acids are catabolized to pyruvate, oxaloacetate, or precursors of these. • Muscle proteins may break down to supply amino acids. • These are transported to liver where they are deaminated and converted to gluconeogenesis inputs. E. Conversion of propionate into succiny1coA: • Propionate enters the main gluconeogenic pathway via the citric acid cycle after conversion to succiny1-coA. • Propionate is first activated with ATP and CoA by an appropriate acy1-CoA synthetase.
- 64. POWER RANGERNOTES FOOD CHEMISTRY 64 • Propiony1 CoA formed undergoes a CO2 fixation reaction to form D- methylmalony1-CoA, catalyzed by propiony1-CoA carboxylase. • This reaction forms a malony1 derivative and requires the vitamin biotin as a coenzyme. • D-Methylmalony1-CoA must be converted to its steroisomer, L-methylmalony1- CoA, by methylmalony1-coA racemase before its final isomerization to succiny1-CoA by the enzyme methlmalony1-CoA isomerase. • It is converted into malate which is then converted into phosphoenol pyruvate and finally to glucose. • Fates of Pyruvate • Three common fates for pyruvate are of prime importance: conversion into acteyl CoA, lactate, and ethanol. ATP production from glucose • Glycolysis • One mole. of glucose → 2 moles of pyruvate • ATP produced = 8 • Pyruvate → Acetyl CoA • ATP produced = 2x3=6 • Citric acid cycle • Acetyl CoA →CO2 + H2O • ATP produced = 12 x2=24
- 65. POWER RANGERNOTES FOOD CHEMISTRY 65 • Net production =30+8=38 Food proteins Chapter 1: Native proteins and denatured proteins 7.1.1.Introduction Proteins are important in foods, both nutritionally and as functional ingredients. They play an important role in determining the texture of a food. Proteins are made up of sequence of amino acids. There are 20 amino acids present in food proteins. 1. Essential amino acids and Nonessential amino acids The body is able to synthesis about 10 amino acids and these amino acids are called non-essential or dispensable amino acids as they can be synthesized in animals from other compounds. They are glycine, cysteine, alanine, serine, proline, tyrosine, aspartic acid, asparagine, glutamic acid and glutamine. The remaining amino acids are called essential or non-dispensable as they cannot be synthesized in animals. They are methionine, tryptophan, threonine, valine, isoleucine, leucine, phenylalanine, lysine, arginine and histidine. Arginine and histidine are essential for infants and children. 2. Limiting amino acid Proteins of different foods have different proportions of essential amino acids. Some of them may contain required mounts of essential amino acid and few of them may not have adequate amounts of one or more of essential amino acids. An essential amino acid of a protein which is present much below requirement is called as limiting amino acid. Most of the plant proteins contain limiting amino acid.
