Lecture 9 (biol3600) genetics of cancer, population genetics
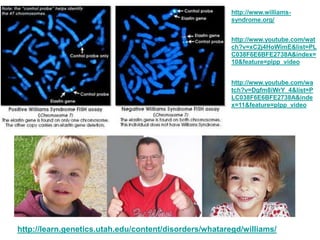
- 1. http://www.williams- syndrome.org/ http://www.youtube.com/wat ch?v=xC2j4HoWimE&list=PL C038F6E6BFE2738A&index= 10&feature=plpp_video http://www.youtube.com/wa tch?v=Dgfm8iWrY_4&list=P LC038F6E6BFE2738A&inde x=11&feature=plpp_video http://learn.genetics.utah.edu/content/disorders/whataregd/williams/
- 2. Site-Directed Mutagenesis • Oligonucleotide-directed mutagenesis
- 4. Knockout Mice • A normal gene of the mouse • has been fully disabled. • Knock-in mice: a mouse carries • an inserted DNA sequence • at specific locations
- 6. Chapter 23 Outline • 23.1 Cancer Is a Group of Diseases Characterized by Cell Proliferation, 638 • 23.2 Mutations in a Number of Different Types of Genes Contribute to Cancer, 642 • 23.3 Changes in Chromosome Number and Structure Are Often Associated with Cancer, 650
- 7. Chapter 23 Outline • 23.4 Viruses Are Associated with Some Cancers, 652 • 23.5 Epigenetic Changes Are Often Associated with Cancer, 653 • 23.6 Colorectal Cancer Arises Through the Sequential Mutation of a Number of Genes, 654
- 8. 23.1 Cancer Is a Group of Diseases Characterized by Cell Proliferation Tumor Formation Cancer as a Genetic Disease The Role of Environmental Factors in Cancer
- 10. Tumor Formation: A Distinct Mass of Abnormal Cells • Benign tumor: the tumor remains localized. • Malignant tumor: tumor cells invade other tissues. • Metastasis: the tumor cells induce secondary tumors.
- 11. Cancer as a Genetic Disease • Genetic evidence for cancer • Carcinogens, chromosomal abnormalities, inheritance • Knudson’s multistep model of cancer • Requires several mutations (ex: retinoblastoma) • The clonal evolution of tumors • Tumor cells acquire more mutations that allow them to become increasingly more aggressive in their proliferate properties.
- 12. Cancer Overview • Cancer is a complex group of diseases that has the ability to affect nearly every tissue and organ in our body • Cancer is a genetic disease 1) Agents that induce DNA mutations also cause cancer (the two go hand in hand) 2) Some cancers are consistently associated with a particular chromosomal abnormality - 90% of CML patient have the chromosome translocation (229) 3) Some forms of cancers run in families - If parents can pass a disease down to offspring, must have a genetic component • Cancer is typically caused by a gradual accumulation of small point mutations in critical genes that control the cell growth, death, and spread - It is never due to a single mutation event - Can involve large chromosomal alterations
- 14. Cancer Overview • Most cancers involve mutations in somatic cells (e.g. skin, liver, colon, lung) rather than gametes - REMEMBER: Mutations in somatic cells can not be passed down to future generations (a smoker with lung cancer will not pass it onto their kids) - Only 1% of cancers involve mutations present in the gamete cells - These mutations do not give the offspring cancer - They only provide offspring with an increased susceptibility to cancer - Such mutations must be accompanied by other mutations once the offspring is born (they get the ball rolling – remember cancer always involves more than one mutation) • Will we ever be able to PREVENT cancer? - NO!! - There will always be some background rate of spontaneous mutation no matter how healthy a person is.
- 16. Role of Environmental Factors in Cancer http://www.newsday.com/news/health/nobel-prize-winner- has-a-family-history-of-scientific-greatness-1.3220104
- 17. 23.2 Mutations in a Number of Different Types of Genes Contribute to Cancer Oncogenes and Tumor-Suppressor Genes Genes That Control the Cycle of Cell Division DNA-Repair Genes Genes That Regulate Telomerase Genes That Promote Vascularization and the Spread of Tumors
- 18. Cancer Overview • Carcinogens greatly increase the rate at which mutations accumulate in our genome - Carcinogen – Any cancer causing agent - Includes alkylating agents, base analogs, radiation (UV and high energy), viruses, intercalating agents, etc. • How come we all don't develop cancer as babies? - DNA repair mechanisms are constantly monitoring our DNA - Checkpoints are in place to give the cell time to repair damage before progressing in the cell cycle - Our immune system has the ability to specifically recognize cancer cells and destroy them As we get older, more mutations accumulate, repair isn't as efficient, and immune system is much less effective as a cancer killer
- 19. Cancer Genes involved • Cancer results from an accumulation of mutations in various functional classes of genes - Includes those involved in the cell cycle, cell death, DNA repair, telomere maintenance, vascularization, and immune evasion • Mutation of genes controlling the cell cycle - First, a review of the cell cycle - Newly-formed cells start in G1 – stay in there until receiving a growth signal to move on - Upon receiving that signal, cells move to S phase (DNA replication) - ONCE IN S, THE CELL MUST DIVIDE!! - Upon finishing DNA rep, cell moves to G2 and then into mitosis At each transition, cells must receive the "ok" signal before it is allowed to move on
- 20. Mutations in cell cycle genes Proto-oncogene vs tumor suppressor • All genes involved in the cell cycle can be divided into 1 of 2 categories 1) Proto-oncogenes - These are genes that normally have a stimulatory effect on the cell cycle - Cells normally only turn them on when they want to progress - Mutations keep them ―on‖ all of the time or turn them ―on‖ at the wrong time - Analogy: Keeping the gas pedal pressed down in your car 2) Tumor suppressors - These genes normally have an inhibitory effect on the cell (keep the cell from moving through the cell cycle if there are problems - Cells turn them ―on‖ when they want to stop - Mutations turn them off permanently - Analogy: Removing the brakes from your car • Mutations involving proto-oncogenes are usually dominant by nature - aa Normal expression, Aa Activated abnormally • Mutations involving tumor suppressors are usually recessive by nature - AA and Aa Normal, aa Inactivated
- 23. Genes That Control the Cycle of Cell Division Control of the cell cycle Cyclin-dependent kinases (CDKs), cyclins G1-to-S transition Retinoblastoma protein (RB) G2-to-M transition Mitosis-promoting factor (MPF) Spindle assembly checkpoint Mutations in cell-cycle control and cancer
- 25. Mutations in cell cycle genes Getting out of G1 • Step 1 in the cell cycle – getting from G1 into S - Growth signals in the environment ultimately tell a cell to either stay in G1 or progress through the cell cycle - General order of events (called signal transduction): 1. Ligand binds to cell receptor 2. Receptor changes following ligand binding 3. Changes in the receptor induces activation of a cytoplasmic protein - Usually via phosphorylation 4. Those proteins then typically activate another protein, which activates another.... 5. Transcription factor(s) is (are) eventually activated Cell either stays in G1 or is given the go ahead to proceed to S
- 27. Mutations in cell cycle genes Getting out of G1 • Why so many steps here???
- 28. Mutations in cell cycle genes Getting out of G1 • Common signal transduction pathways 1) Stimulatory pathways – When activated, tell the cells to proliferate - Overactivation of such pathways leads to TUMOR formation - Inhibition of such pathways leads to cell shut-down - Activated by growth factors and cytokines - End result is to activate proteins called cyclins and CDKs (Cyclin-Dependent Kynases) - These are the proteins that directly function to propel the cell through the cell cycle 2) Inhibitory pathways – When activated, keep the cell from moving out of G1 - Over-activation of such pathways leads to further cell shut-down - Inhibition of such pathways can lead to CANCER - Keeps the cell from dividing when it shouldn’t be dividing - Includes proteins that directly inhibit the function of cyclins and CDKs
- 29. Mutations in cell cycle genes Progression through the cell cycle • A variety of transcription factors, kinases, phosphatases, and ubiquitinating enzymes control the expression and activity of cyclins and CDKs - Proteins that inhibit cyclin/CDK activity (directly/indirectly) 1) CDK inhibitory proteins (CIPs) - These proteins bind to CDK CDK2 proteins and block their CDK2 Progression activity - Keep the cell from moving on cyc A cyc A CIP X to S phase in the cell cycle - Key CIPs include p21 and p16 DNA p53 damage 2) p53 - DNA damage activates p53 - Activated p53 moves into the nucleus and activates transcription of other inhibitory genes (e.g. CIPs) - DNA damage p53 activated CIPs produced CDKs/cyclins blocked cell fails to progress
- 30. Mutations in cell cycle genes Progression through the cell cycle Rb 3) Rb Rb - A major transcription factor that initiates Rb E2F E2F cyclin and CDK production is called E2F E2F - Rb binds to prevents E2F activation X Activation of - These are just a few of the many proteins that function cyclins/CDKs to keep the cell from progressing through the cell cycle when the time is not right or there is some kind of cell damage - CIPs, p53, and Rb are all considered tumor suppressors because they naturally function to prevent the cell from dividing - Inactivation of such genes commonly leads to abnormal cell growth (removing the brakes of the car) - Over 90% of human cancers have defective p53 or Rb activity - Some viruses target these proteins [HPV E6 (p53) and E7 (Rb)] http://www.youtube.com/watch?v=GnvQ9qsrMRU
- 31. Mutations in DNA repair genes Cancer requires lots of mutations • Cancer arises from the accumulation of various mutations within a single cell - How do cancer cells acquire so many mutations? What about the repair pathways? • Two factors control the rates at which mutations are introduced into the DNA 1) Rate at which errors arise during the course of replication: - Is the proofreading mechanism of DNA pol working properly? - Not generally affected in cancer cells 2) Efficiency with which errors are repaired - Mutation in the different repair pathways can lead to a massive accumulation of point mutations and chromosomal rearrangements. - Analogy: Like shutting down the police to allow increased crime • Repair of small DNA lesions - Many forms of cancer have been linked with defects in NER, BER, and mismatch repair • Defects in pathways that repair breaks in DNA strands can lead to large chr. rearrangements (deletions, inversions, translocations, etc.) CANCER
- 32. Mutations in cell death genes ―A time to live, A time to die‖ • Cells are living entities that at some point must die - They usually die as a result of injury or as a result of cell suicide (apoptosis) • Cell death by injury (unplanned) - NECROSIS - Caused by mechanical or chemical damage - Plasma membrane transport is disrupted and the cell (and all organelles) begin to swell - The cell ruptures, leaking all its cytoplasmic components into the surrounding tissue - Analogy: Explosion/burns - CAUSES INFLAMMATION very bad • Programmed cell death – APOPTOSIS - Cell shrinks, plasma membrane forms blebs, DNA is fragmented, cell is fragmented into little vesicles (called apoptotic bodies) - Immune cells engulf the apoptotic bodies - NO INFLAMMATION (Analogy: Implosion)
- 33. Mutations in cell death genes ―A time to live, A time to die‖ • What tells a cell to kill itself via apoptosis? 1) Signals from outside the cell - Virally-infected and cancerous cells will be induced to kill themselves by cells of the immune system called natural killer cells and cytotoxic T cells - Immune cells interact with receptors on the surface of these altered cells and initiate an apoptotic pathway Immune system tells the cell to kill itself 2) Signals from inside the cell - Extensive DNA damage or other cellular issues (e.g. aging, malfunctioning) will initiate an internal apoptosis pathway involving mitochondria Cell kills itself on its own • Apoptosis (in both cases above) is very tightly regulated by a number of genes - Some function to prevent the induction of apoptosis when everything looks normal (anti-apoptotic) and others regulate the actual events of the process (pro-apoptotic) - Alteration of these genes can lead to apoptosis at the wrong time (e.g. autoimmune disorders, neurodegenerative disorders) or not at all (CANCER)
- 34. Mutations in genes controlling telomerase Keeping cancer cells growing indefinitely • Review of mutations discussed so far: 1) Cell cycle control - Abnormal activation of stimulatory or abnormal inhibition of inhibitory proteins causes cells to divide uncontrollably 2) DNA repair - Inhibition of DNA repair mechanisms leads to the gradual accumulation of mutations 3) Apoptosis - Abnormal activation of anti-apoptotic genes or inhibition of pro-apoptotic genes causes cells to stay alive despite having severe genetic problems The above mutations would be pointless if the cell still ends up dying as a result 5’ 3’ 3’ 5’ of shortened telomeres 5’ 3’ 3’ 5’ • Remember: The end of chromosomes (telomeres) shorten after every round of DNA replication b/c DNA polymerases can't fill in the space after the RNA primers are removed from the absolute ends of the chromosome
- 35. Mutations in genes controlling telomerase Keeping cancer cells growing indefinitely TAGGGTTAGGGTTAGGG • If nothing were done to correct for this gradual shortening, cells would die after only a few GGGTTAGGG replication cycles b/c critical genes would be lost • Normal situation - In sperm, eggs, and early embryos, an enzyme called telomerase is activated and adds 1000s of copies of a repeated garbage sequence onto the telomeres cell - That garbage is gradually shortened for the life of the death organism (b/c of repeated cell divisions) - It eventually runs out, ―good‖ chromosomal sequence is lost, and the cell dies - Cells have a limited life span b/c of telomere shortening Telomerase is normally only turned-on in gametes, embryos, and stem cells • Cancer - Estimated that ~90% of cancer cells have abnormally activated telomerase - Telomeres never shorten, cell never ―ages‖, cell does not die (IMMORTAL) - Thought to occur early in cancer formation – TARGET FOR THERAPY??
- 36. 23.3 Changes in Chromosome Number and Structure Are Often Associated with Cancer • Chromosomal instability is a general feature of cancer cells • Deletions, inversions and translocations • Example: A reciprocal translocation between chromosome 9 and 22 causes chronic myelogenous leukemia. • Aneuploidy
- 40. Mutations in genes controlling tumor spread Angiogenesis and metastasis • Tumor cells need a blood supply (nutrients and oxygen) just like any other cells in our body - Abnormal, large masses of cells (tumor) forming in our tissue initially lack a blood supply and thus grow relatively slowly • At some point, cancer cells acquire mutations in genes that promote the growth of new blood vessels - Growth of new blood vessels is called angiogenesis - Cancer cells begin abnormally releasing pro-angiogenesis factors into the surrounding environment (and block natural angiogenesis inhibitors) - Examples of such factors include vascular endothelial cancer cell growth factor (VEGF), basic fibroblast growth factor bFGF), and angiogenin VEGF or bFGF • When such factors reach an existing blood vessels, they activate endothelial cells within the blood vessel wall blood vessel endothelial cells http://www.youtube.com/watch?v=acUl9JC70e8
- 41. Mutations in genes controlling tumor spread Angiogenesis and metastasis • Activated endothelial cells begin dividing and producing proteins that help to degrade the extracellular matrix (ECM) - Review: Extracellular matrix is a complex network of proteins and carbohydrates that help to protect the cell and keep them anchored into the tissue - Proteins that degrade the ECM are called matrix metalloproteinases (MMPs) - Breakdown of the ECM by MMPs permits new endothelial cells to migrate in the tissue (towards activated an injury site or tumor) endothelial cell MMP • As they move, the endothelial cells eventually form a hollow tube that will be the starting point for a new blood vessel • Once tumor cells recruit blood vessels, 2 things can happen 1) Increased rate of growth (not as bad) 2) Tumor cell invasion into the blood stream (metastasis)
- 42. Mutations in genes controlling tumor spread Angiogenesis and metastasis • In theory, blocking cancer cells from acquiring a blood supply should lead to tumor death - Nearly 20 different angiogenesis inhibitors are currently being tested for their ability to block tumor formation - They work at different points in the angiogenesis pathway - Release of pro-angiogenesis factors by tumor cells, endothelial cell replication and signaling, MMP activity - Problem: What about normal angiogenesis (following tissue damage) • Malignancy and metastasis - Tumor cells don’t necessarily have to rely on blood vessels to come to them in order to move - They can acquire mutations that allow them to move into surrounding tissues - Many tumor cells will begin producing their own MMPs in order to break through the ECM - Allows them to invade tissue (malignant) and spread to distant sites (metastatic)
- 43. Tumor immune evasion Killing and fooling the protectors • Our immune system is very efficient at recognizing and killing tumor cells (normally) - Uses the Fas-FasL system of apoptosis • If tumor cells weren’t bad enough up to this point, they often acquire the ability to either fool the immune system into thinking they are normal, or they actively kill the immune cells • Some examples of immune evasion by tumors 1) Production of decoy Fas by tumor cells - Activated immune cells express Fas ligand - Binds to Fas on tumor cells and induces apoptosis - Some tumor cells release a soluble form of Fas - This will bind to Fas ligand on the immune cell and block it from interacting with Fas on the cancer cell itself 2) Killing the killers - Some tumor cells will express high levels of Fas ligand - Immune cells express Fas - Binding induces apoptosis in the immune cell instead of the tumor cell
- 44. Cancer Review • We have covered a variety of genetic changes that are observed in the formation of cancer 1) Those that promote progression through the cell cycle 2) Those that block DNA repair 3) Those that block apoptosis 4) Those that activate the expression of telomerase 5) Those that increase rates of transcription and translation 6) Those that promote vascularization and spread of tumor cells 7) Those that help tumor cells evade the immune system • This is just the tip of the iceberg - In each of the categories described, there are probably 10-20 ways tumor cells can alter normal pathways • Each type of tumor does things in a unique way - Lung cancer vs. pancreatic cancer in the same person Very different - Lung cancer vs. lung cancer in two different people Very different Universal treatment for all cancers may be difficult (very difficult!) to achieve
- 45. Population genetics Assigned reading: Chapter 25
- 46. Chapter 25 Outline • 25.1 Genotypic and Allelic Frequencies Are Used To Describe The Gene Pool Of a Population, 694 • 25.2 The Hardy-Weinberg Law Describes the Effect of Reproduction on Genotypic and Allelic Frequencies, 697 • 25.3 Nonrandom Mating Affects the Genotypic Frequencies of a Population, 701 • 25.4 Several Evolutionary Forces Potentially Cause Changes in Allelic Frequencies, 704
- 47. Population genetics Overview 1) NATURAL SELECTION HAPPENS! EVOLUTION proceeds with natural selection acting on variation (WHY? Some of the variations survive!) 2) Variations happen within individuals 3) Variations might give an individual some form of reproductive advantage. (ex: bigger/stronger/faster/better-looking, etc… Although many don’t have a lot of those advantages and still do fine!) 4) Selection for passing on genes. 5) When individuals are selected, the population changes (…when Darwin met Mendel) When population changes (Darwin), we get variation (Mendel) Darwin + Mendel = understanding of evolution
- 48. Population genetics Overview ~1930s the notion of Population Genetics started to emerge: • scientists wanted to predict the gene frequency and genotype frequencies in a non-evolving population this is what the Hardy-Weinberg equation talks about gene pools and collection of individuals Population = all the individuals of the same species confined in the same area • Looking at frequency of genotypes: Qt: What is genotype frequency? Is the proportion or percentage in which a particular genotype shows-up in a population: • Looking at a locus (2 alleles) that has 3 genotypes: AA, Aa and aa the frequency (f) of each genotype is: f(AA) = number of AA individuals N
- 49. Population genetics Overview • Looking at a locus (2 alleles) that has 3 genotypes: AA, Aa and aa the frequency (f) of each genotype is: f(AA) = number of AA individuals N f(Aa) = number of Aa individuals N f(aa) = number of aa individuals N THE SUM OF ALL GENOTYPIC FREQUENCIES ALWAYS EQUALS TO 1. f(AA) + f(Aa) + f(aa) = 1
- 50. Genetic variation Calculating genotypic frequencies • Calculating genotypic frequencies - Example above – what percentage of the individuals in the population are AA, Aa, or aa - To do this, one would take a sample of the population, calculate the frequencies, and use statistics to demonstrate significance of the findings - To calculate the genotypic frequency: Example f(AA) = (number of AA individuals)/N f(AA) = 500/ 1000 = .5 f(Aa) = (number of Aa individuals)/N f(Aa) = 290/1000 = .29 f(aa) = (number of aa individuals)/N f(aa) = 210/1000 = .21 where N represents the number of individuals tested f(AA) + f(Aa) + f(aa) = 1 ALWAYS!!
- 51. Population genetics Overview • When looking at a locus (2 alleles) it’s easier to calculate allelic frequencies, since there are always fewer alleles than there are genotypes. Alleles: A or a the frequency (f) of each allele is: f(Allele) = number of copies of the allele number of copies of all alleles at the locus p = f(A) = 2n AA + n Aa 2N q = f(a) = 2naa + n Aa 2N where nAA, nAa, naa represent the number of individuals who are AA, Aa, and aa, respectively, and N is the total number of individuals tested. (dividing by 2N because there are 2 alleles at a locus) p + q = 1 ALWAYS!!
- 52. Population genetics Overview ~1930s the notion of Population Genetics started to emerge gene pools and collection of individuals Population = all the individuals of the same species confined in the same area • Now… If there is no individual change, then the genes frequency should never change. • Looking at frequency of alleles: AA x aa A=50%; a=50% • First generation: all Aa A=50%; a=50% • Second generation: (do it on board) A= __%; a=__% (we are talking about the population here! Also: random mating = everyone mates with everyone else!)
- 53. Genetic variation Calculating allelic frequencies • Now we can use laws of probability to predict genotypes from a knowledge of gene frequency. • The frequency of the dominant allele is equal to ―p‖ • The frequency of the recessive allele is ―q‖ • If we have 2 alleles, what is the probability of combinations we can possibly have at one locus? p2 + 2pq + q2 = 1 (we have seen that before…)
- 54. Genetic variation Calculating allelic frequencies p2 + 2pq + q2 = 1 • Now for the practical predictions: Sample question: In a sampling of 20,000 people of Eastern European Jewish descent in a particular city (Ex: Ft. Lauderdale), there were 2 individuals born with the disease. Determine the number of carriers for Tay-Sachs disease in that group.
- 55. Genetic variation Calculating allelic frequencies Qt: In a sampling of 20,000 people of Eastern European Jewish descent in a particular city (Ex: Ft. Lauderdale), there were 2 individuals born with the disease. Determine the number of carriers for Tay-Sachs disease in that group. A: Tay-Sachs is a recessive disease, so the frequency of ―aa‖ is 2/20,000 = 0.0001 getting to our equation: p2 + 2pq + q2 = 1 q2 = 0.0001 ….so q = 0.01 Now go get the ―p‖ p + q = 1 p = 0.99 Now figure-out the number of carriers: p2 + 2pq + q2 = 1 Carriers = heterozygous individuals = 2pq 2pq = 2(0.99)(0.01) = 0.02 this means that 2% of the population is heterozygous. If your total population number is 20,000, then 2% of 20,000 is 400.
- 56. Genetic variation Calculating allelic frequencies • Calculating allelic frequencies - Allelic frequencies can be calculated in two ways depending on whether or not the genotypic frequencies are known: 1) If the genotypic frequencies have not been calculated - Example: Imagine that I want to determine the allelic frequency of the 2 codominant alleles of the MN blood antigen gene in a specific population Phenotype Genotype Number of individuals MM LMLM 182 MN LMLN 172 NN L NLN 44 p = f(A) = 2n AA + n Aa 2N p = f(LM) = [2 (# of LMLM) + (# LMLN)]/2(398) = [2(182) + 172]/796 = 536/796 = .673 q = f(LN) = 2 (# of LNLN) + (# LMLN)]/2(398) = [2(44) + 172]/796 = 260/796 = .327
- 57. Genetic variation Calculating allelic frequencies • Calculating allelic frequencies - Allelic frequencies can be calculated in two ways depending on whether or not the genotypic frequencies are known: 2) If the genotypic frequencies HAVE been calculated p= f(A) = f(AA) + ½ f(Aa) q= f(a) = f(aa) + ½ f(Aa) - Same example: Phenotype Genotype Number of individuals MM LMLM 182 MN LMLN 172 NN LNLN 44 Genotypic frequency f(LMLM)= 182/398 = .457 f(LMLN) = 172/398= .432 f(LNLN) = 44/398=.111 p = f(A) = .457 + .5(.432) = .673 q = f(a) = .111 + .5(.432) = .327
- 58. Genetic variation Calculating allelic frequencies • Calculating allelic frequencies with more than 2 alleles (it can be done! But you will not be tested on it!) - Use same type of formulas, except they must account for more alleles - Example: A gene contains three alleles A1, A2, A3 2nA1A1 + nA1A2 + nA1A3 p = f(A1) = 2N = f(A1A1) + ½ f(A1A2) + ½ f(A1A3) 2nA2A2 + nA1A2 + nA2A3 q = f(A2) = 2N = f(A2A2) + ½ f(A1A2) + ½ f(A2A3) 2nA3A3 + nA1A3 + nA2A3 r = f(A3) = 2N = f(A3A3) + ½ f(A1A3) + ½ f(A2A3) In this case, p + q + r = 1
- 59. Hardy-Weinberg Law Definition and assumptions • Question: Will a dominant allele that is currently rare in the population gradually increase in frequency over time? - If an allele has a frequency of 0.05 today, will it be 0.10 20 years from now? • Such questions led two scientists to independently come up with a mathematical model to explain what happens to allelic (and genotypic) frequencies over time - This model is called the Hardy-Weinberg law • Hardy-Weinberg law - Examines the effect of reproduction ALONE on allelic and genotypic frequencies - It assumes that other factors (variables) will not be affecting allelic frequencies - Assumptions are as follows: 1) Individuals of all genotypes have equal rate of survival and equal reproductive success – THERE IS NO SELECTION 2) No new alleles are created or changed by mutation
- 60. Hardy-Weinberg Law Definition and assumptions • Hardy-Weinberg law 3) Individuals do not migrate or move out of the population 4) The population is very large - Large enough that sampling errors are negligible 5) Individuals in the population mate randomly - Mathematically, this means that each genotype mates in proportion to its frequency - These assumptions don’t have to apply for all genes of the organism - The Hardy-Weinberg law only applies to a single locus (one gene at a time)
- 61. Hardy-Weinberg Law Example Allelic frequencies f( )= 3/10 = .3 generation 1 f( ) = 7/10 = .7 Genotypic =.7 x .7 = .49 =.3 x . 7 = .21 frequencies =.3 x .3 = .09 =.7 x .3 = .21 in gen. 2 Allelic frequencies generation 2 f( ) = .49 + ½(.42) = .7 f( ) = .09 + ½(.42) = .3 - In an ―ideal‖, unchanging population, allelic frequencies do not change from generation to generation as a result of reproduction - What would happen if 4 greens were added in? Allelic frequencies would change (Does not meet the assumptions)
- 62. Hardy-Weinberg Law Predictions • Hardy-Weinberg law - If the above assumptions are met (if you have an ideal large population with no selection, migration or mutation), the Hardy-Weinberg law predicts: 1) The frequency of alleles does not change from generation to generation - If p=.6 in one generation, it will still be .6 after 5 generations 2) Genotypic frequencies stabilize and remain constant after 1 generation of random mating - They stabilize in the proportions of p2 (AA), 2pq (Aa), q2 (aa) - At this point, the population is said to be at Hardy-Weinberg equilibrium - Overall, the Hardy-Weinberg law predicts that reproduction alone DOES NOT alter allelic frequencies - Alleles just keep segregating (meiosis) and recombining (fertilization) with no real change in allelic frequencies - No allele has an ―advantage‖ over another – they all stay in the same ratios - Evolution can not take place if a population is in Hardy-Weinberg equilibrium - Other factors (mutation, selection, migration) must be present to alter allelic frequencies from generation to generation
- 63. Nonrandom mating Overview • Remember: An unchanging, isolated population in Hardy-Weinberg equilibrium must be large, randomly mating, and unaffected by mutation, migration, or natural selection - One or more of these conditions are often not met in real populations - Sometimes mating is not random, sometimes genes acquire mutations (new alleles are formed), sometimes individuals come into and leave populations, ... • Nonrandom mating - Nonrandom mating alone does not alter allelic frequencies, but it can influence genotypic frequencies - There are two broad classes of nonrandom mating: 1) Positive assortative mating - This is the tendency for like individuals to mate - Example: Humans generally exhibit positive assortative mating for height (all other loci are still being distributed randomly) - Tall people prefer to mate with other tall people, short with short 2) Negative assortative mating - Opposites attract (Example: Would be if tall preferred to go with short)
- 64. Nonrandom mating Inbreeding • A common form on nonrandom mating is inbreeding, which is the preferential mating between related individuals - Positive assortative mating for relatedness - Unlike other examples of assortative mating, inbreeding affects all genes and not just those that determine the trait for which the preference exists (e.g. height) • Since it is a form of nonrandom mating, inbreeding causes a deviation from the Hardy-Weinberg equilibrium - Inbreeding leads to an increase in the proportion of homozygotes and a decrease in heterozygotes - f(AA) and f(aa) go up, f(Aa) goes down • Inbreeding is usually measured by the inbreeding coefficient (F) - F = measure of the probability that two alleles are identical because they are descended from the same single A
- 65. Nonrandom mating Inbreeding • Inbreeding coefficient (F) - Ranges from 0-1 - F=0 means that no individuals have any alleles derived from a common ancestor (mating is random) - F=1 means that all alleles are identical by descent (all individuals are homozygous) • Effect of inbreeding on heterozygosity - Again, inbreeding leads to an increase in homozygotes and a decrease in heterozygotes • Genetic/physical effects of inbreeding - Inbreeding is typically harmful because it increases the likelihood that two harmful or lethal recessive alleles will get together - Harmful effects previously concealed in heterozygotes - Increased appearance of harmful and lethal alleles as a result of inbreeding is called inbreeding depression - Greater the inbreeding, more severe the inbreeding depression
- 66. Nonrandom mating Inbreeding • Examples of inbreeding depression 1) Schull and Neel study of Japanese children - For each 10% increase in F, the mean IQ of children dropped 6 points - Inbreeding also leads to an increase of child mortality - Children of first cousins are 40% more likely to die in the first few years than children of unrelated people 2) Inbred mice - Scientists created 2 strains of mice – those that had no inbreeding and those that had a F=.25 - When both were released into the wild, the weekly survival rate of the inbred mice was only 56% of that of non-inbred mice • Selected inbreeding (e.g. domesticated animals and plants) can be used to generate organisms with desirable traits - If done properly, can weed out harmful recessive mutations
- 67. Processes that alter allelic frequencies Mutation • Nonrandom mating produces changes in genotypic frequencies, but has no effect on allelic frequencies - The same alleles are there – they are just getting reshuffled • Other processes can cause individual alleles to increase or decrease in frequency - These include mutation, migration, genetic drift, and natural selection • Mutation - The only process in nature that creates NEW alleles is mutation - The other processes just reshuffle existing alleles - Mutations occur randomly, slowly, and with no regard for whether it will be beneficial or harmful for the organism - Mutation can have a small, but significant effect on allelic frequency - Mutation can convert 1 allele to another (e.g. from p to q or q to r) - The effect of mutation on allelic frequency depends on the mutation rate and the difference between p and q
- 68. Processes that alter allelic frequencies Mutation • Mutation - Example: - Two alleles have starting frequencies of p=.9 and q=.1 - Since there are more p alleles, there are more pq mutations than qp mutations - Such mutations gradually increase the frequency of q - This will slightly increase the rate of qp mutation and decrease the rate of pq mutation - At some point, enough p will be converted to q such that the rates of mutation of pq and qp will be equal - At this point, the allelic frequencies will be stabilized and at equilibrium This effect is similar to what occurs during a chemical reaction (reactprod) or diffusion (think about it)
- 69. Processes that alter allelic frequencies Migration • Migration or gene flow is the movement of organisms/genes/alleles into or out of the population - Migration is a normal process in most natural populations - It prevents genetic divergence between populations (makes them more similar) and increases genetic variation within populations • Effect of migration on allelic frequencies - Example: A certain subset of 1 population migrates to a second population - Magnitude of changes in allelic frequencies in the recipient population depends on two factors: 1) Extent of migration - How many individuals are migrating? - Greater the migration, greater the change in allelic frequency in the recipient population 2) Difference in allelic frequency between the source and recipient populations - Greater the starting difference, greater the change in recipient - If the 2 populations have the same freq., the two are in equilibrium (no Δ)
- 70. Processes that alter allelic frequencies Genetic drift • The Hardy-Weinberg equilibrium assumes random mating in an infinitely large population - Real populations are often limited in size - Small populations are at a greater risk of having major changes in allelic frequencies (genetic drift) from generation to generation. • Genetic drift is a chance event - Chance influences what alleles will be present in the limited sample of the population
- 71. Processes that alter allelic frequencies Genetic drift • Causes of genetic drift (factors that effectively create a small population) - There are several different ways in which sampling error can arise and lead to genetic drift 1) Limitations in space, food, or other resources lead to a reduction in population size - What is left over may randomly be very different than the original 2) Founder effect - A small group leaves one population and establishes a new population - The alleles carried by that small group will determine the genetic make-up of the new population - The alleles carried by the founders may not be representative of the original population - Chance determines who the founders will be 3) Bottleneck effect - A population undergoes a drastic reduction in population size (catastrophe) - The remaining survivors will determine the genetic make-up of the population
- 72. Processes that alter allelic frequencies Genetic drift • The smaller the sample taken, the greater the potential for changes in allelic frequency when a new population grows - Example: What if I flip a coin just twice? • Effects of genetic drift on allelic frequencies 1) Genetic drift leads to a random change in allelic frequencies within a population - Frequencies are equally likely to increase or decrease 2) Genetic drift often leads to reduced genetic variation within a population - Example: In a starting population, the frequencies of two alleles A and a for a given locus are f(A) = p = .7 and f(a) = q = .3 - If that population is decimated by disease and only 10 members remain, it is possible that those 10 members would all be AA - In this case, the a allele has been lost forever in this population and will not reenter the population unless there is mutation or migration - The A allele is said to be fixed in the population - More common the allele the more likely it is to be fixed
- 73. Processes that alter allelic frequencies Genetic drift • Effects of genetic drift on allelic frequencies 3) Genetic drift can cause different populations to diverge over time - Since genetic drift is a random event, it makes sense that 5 different populations under the influence of genetic drift may change in different ways - Some will be fixed for a given allele (A), others will be fixed for the other allele (a), others will not - This causes populations to naturally diverge from one another with respect to allelic frequencies
- 74. Processes that alter allelic frequencies Natural selection • Natural selection is the differential reproduction and/or survival of individuals in a population due to genetic differences - Occurs when individuals with adaptive traits produce more offspring than other members of the population - If the adaptive traits have a genetic basis (in the germline), the offspring will also express those traits (and they will also produce lots of offspring) - Over time, the frequency of the ―beneficial‖ allele will gradually increase in the population (may become fixed) Such selection promotes adaptation • Effect of natural selection on the gene pool depends on the fitness levels of the different genotypes - Fitness = RELATIVE reproductive success of a genotype - Genotypes associated with high levels of reproductive success have high fitness whereas those having low reproductive success have low fitness - Fitness (W) ranges from 0 – 1, with 1 being the genotype with the highest level of fitness
- 75. Processes that alter allelic frequencies Natural selection • Results of selection - There are three broad types of selection based on the outcome on the population (1) Stabilizing selection - The intermediate phenotype is favored and will be selected for - Example: Human birth weight. Overly large babies may kill the mother, small babies may not survive - This is a case in which WAA<WAa>Waa - Neither allele will ever be eliminated from the population because the heterozygote contains both alleles (and is favored) - If WAA is different from Waa, may see a change in allelic frequencies initially - Over time, a stable equilibrium will be reached - At this point, no further change in allelic frequencies will be observed (unless migration, mutation, c-section.....)
- 76. Processes that alter allelic frequencies Natural selection • Results of selection - There are three broad types of selection based on the outcome on the population (2) Disruptive selection - This is a case where the intermediates (Aa) are selected against (both homozygotes are favored) - WAA>WAa<Waa - Leads to an unstable equilibrium (any little changeFixation) - Example: Predator and prey size - Prey that are too small are hard to find - Prey that are too large are hard to kill - Intermediate sized prey are killed off (low fitness) (3) Directional selection - This is selection in favor or against one of the extremes (e.g. AA or aa) - WAA>WAa>Waa or Waa>WAa>WAA - Leads to gradual change in allelic frequency (no real equilibrium) - Example: Peppered moths
- 77. Evolutionary genetics Assigned reading: Chapter 26
- 78. Evolutionary genetics Overview • The various processes discussed last lecture (mutation, natural selection, genetic drift) can all cause species to gradually change into a different species or split into multiple, distinct species - Such changes are what constitute evolution - Evolution = Genetic based change in a group of individuals over a number of generations • In the past, how would we determine evolutionary relationships between species? - Physical traits – do they look different, eat different things, live in different areas, etc. - Not good b/c it creates too much gray area 1) How does one quantitate phenotypic differences 2) Physical traits may be determined by a single gene or many different genes - Makes analysis of trait differences very difficult 3) Environment often affects physical traits (NOT EVOLUTION) - Example: Two flowers with the same genotype may have different color flowers because of differences in the pH of the soil - They are identical twins, but look different b/c of the environment
- 79. Evolutionary genetics Using a molecular approach • Issues just described led scientists to begin using molecular differences (protein and nucleic acid sequences) as a means of analyzing evolutionary change • Molecular data offer a number of advantages over traditional methods for studying evolution 1) Molecular data are genetic and more accurately measure evolution - Evolution is caused from genetic changes over time - Anatomical, behavioral, and physiological traits may or may not be due to actual genetic changes - Example: If I find a lizard skeleton that is missing a tail and one that has a tail, could I say if they are members of different species (what if the lizard lost its tail in an accident) - DNA doesn’t lie 2) Molecular methods can be used for all organisms - Everything on the planet has DNA and protein - Can compare any two species on the planet and see how closely ―related‖ they are - How different is a human from an elephant vs. a rhinoceros
- 80. Evolutionary genetics Using a molecular approach • Molecular data offer a number of advantages over traditional methods for studying evolution 3) Large amounts of molecular data can be obtained and compared - Can examine every gene from our genome (3 billion bp) and compare it to those genes from a rhino - Not restricted to looking at genes that code for physical traits 4) Molecular data are quantifiable - Can objectively, accurately, and precisely analyze molecular data - Example: Can say that the cyt b gene from humans and zebrafish have exactly 23 nucleotide differences - Using physical characteristics, how could you ever come up with a number to represent the difference between two species (human and chimp) 5) Molecular data provide clues about the process of evolution - If two species have look similar but have different feeding habits, you can analyze the DNA/protein sequences and see exactly what genetic differences led to the different behaviors
- 81. Evolutionary genetics Protein variation • DNA and protein sequence analysis began being used for population and evolutionary studies soon after electrophoresis was invented - Electrophoresis – Use of an electric current to separate macromolecules based on some physical characteristic (e.g. size, charge, isoelectric point) • Proteins were characterized long before DNA - Early molecular studies were aimed at examining differences in proteins between members of the same population, between different populations of the same species, or between different species - 1966 – Scientists extracted proteins from different members of a population and separated them via gel electrophoresis - Different banding patterns = different genotypes
- 82. Evolutionary genetics DNA sequence variation • Techniques developed in the last 25 years have allowed us to directly analyze DNA sequences and measure genetic variations between individuals and species • There are several ways in which DNA sequences have been used for these purposes: 1) RFLP analysis – Earliest way of comparing DNA sequences - RFLP – restriction fragment length polymorphisms (aka differences) - Differences in sequence between 2 pieces of DNA often lead to differences in restriction enzyme cut sites - Greater the RFLPs Greater the differences in the sequence Greater the genetic variation - Not ideal – what if genetic variation is great, but does not happen to alter any restriction enzyme sites 2) PCR of microsatellites - Microsatellites are short sequences that are repeated in tandem (aka short tandem repeats) 3) Direct sequencing of SNPs and other polymorphisms
- 83. Evolutionary genetics DNA sequence variation • There are several ways in which DNA sequences have been used for these purposes: 3) Direct sequencing of DNA - This is what you did in lab (sort of) - Sequence pieces of DNA in question - Align the sequences using an appropriate computer program - Create an evolutionary (phylogenetic) tree which visually illustrates how genetically similar/distant individual DNA sequences are from one another - Example: Dentist with HIV - In 1990, a woman who had no risk factors came down with HIV - She contracted it from her dentist, who knew he had the disease a few years back (had AIDS) - CDC found 10 of the dentist’s former patients had HIV WHO GOT IT FROM HIM?
- 84. Evolutionary genetics Phylogenetic trees – Sequence alignment • Phylogenetic trees are usually created by comparing individual gene sequences (e.g. cytochrome c) rather than comparing whole organisms • When comparing genes from a set of 5 species, phylogenetic trees may differ depending on: 1) The gene chosen to study - One gene may be 99% identical between 2 species, whereas a different one may be only 42% identical 2) How the sequences are aligned - There is more than 1 way to align a set of sequences - Different alignments Different looking trees • Consider the following alignment: Gene X from species A: 5’—ATTGCGAA—3’ Gene X from species B: 5’—ATGCCAAC—3’ Alignment 1: 5’—ATTGCGAA—3’ Alignment 2: 5’—ATTGCGAA—3’ 5’—ATGCCAAC—3’ 5’—AT--GCCAAC—3’ Computer programs assess the likelihood of each of the changes
- 85. Evolutionary genetics Molecular clock • If we assume that a given region of DNA or protein is changed at a constant rate over time, then the rate of change that a given protein undergoes can be used as a molecular clock • Example of a molecular clock: - If we align cytochrome c sequences from a specific species of alligator with a species of crocodile and find that the sequences have 20 amino acid substitutions - Imagine fossil evidence tells us these two species diverged from a common ancestor 400 million years ago - From this, we know ~ 5 amino acid subs occur every 100 million years - Imagine I now compare cytochrome c from 2 completely different species and find that they have 15 amino acid subs - No fossil evidence exists. When did they diverge? We know that ~5 amino acid subs occur per 100 million years in cyt c If these two differ by 15 amino acids, estimate that they diverged 300 mya
