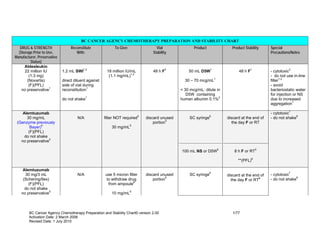
CHEMOTHERAPY PREPARATION AND STABILITY CHART
- 1. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Aldesleukin 22 million IU 1.2 mL SWI1,2 18 million IU/mL 48 h F1 50 mL D5W1 48 h F1 - cytotoxic3 (1.3 mg) (1.1 mg/mL)1,2 - do not use in-line (Novartis) direct diluent against 30 – 70 mcg/mL1 filter1,2 (F)(PFL) side of vial during - avoid no preservative1 reconstitution1 < 30 mcg/mL: dilute in bacteriostatic water D5W containing for injection or NS do not shake1 human albumin 0.1%2 due to increased aggregation1 Alemtuzumab - cytotoxic7 5 6 30 mg/mL N/A filter NOT required discard unused SC syringe discard at the end of - do not shake8 (Genzyme previously portion5 the day F or RT Bayer)4 30 mg/mL5 (F)(PFL) do not shake no preservative5 100 mL NS or D5W5 8 h F or RT5 **(PFL)8 Alemtuzumab 30 mg/3 mL N/A use 5 micron filter discard unused SC syringe6 discard at the end of - cytotoxic7 (Schering/Ilex) to withdraw drug portion9 the day F or RT8 - do not shake8 (F)(PFL) from ampoule8 do not shake no preservative9 10 mg/mL9 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 1/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 2. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) 100 mL NS or D5W9 8 h F or RT8 **(PFL)8 Amifostine 500 mg 9.7 mL NS only10 50 mg/mL10 24 h F, 5 h RT10 25–50 mL*NS only10 5–40 mg/mL: - noncytotoxic10 (MedImmune) 24 h F,10 5 h RT - discard cloudy (RT) solution11 no preservative10 Amsacrine 75 mg/1.5 mL glass syringes 5 mg/mL12 24 h RT12 500 mL D5W12 7 d F, 48 h RT12,13,14 - cytotoxic15 (Erfa Canada) preferred during (RT) reconstitution; PFL12 (plastic or glass no preservative12 max. time in plastic container)12 syringe12: 15 min 13.5 mL supplied diluent (L-lactic acid)1 transfer 1.5mL from ampoule into the diluent vial12 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 2/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 3. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Asparaginase16 (asparaginase E. coli) do not shake; roll to 2500 units/mL16 48 h F, RT16 syringe16 14 d F20, 16 - cytotoxic3 10,000 units reconstitute18,11 (Orphan 4 mL SWI19 Pharmaceutical Intradermal test11: International) Reconstitute with (F) 5 mL SWI to give no preservative17 2000 units/mL Transfer 0.1 mL to 10 mL vial (or 12 mL syringe) Add 9.9 mL SWI roll to dissolve to give 20 units/mL 2 unit test dose = 0.1 mL (Note: the rest of the reconstituted vial has a concentration of 2000 units/mL)11 50 mL*NS or D5W20,13 14 d F,20,13 2 d RT20,21 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 3/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 4. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Erwinia asparaginase (asparaginase Erwinia do not shake; roll to 10000-5000 15 min in original glass or 8 h in a glass or - cytotoxic3 chrysanthemi) reconstitute22 units/mL container; 8 h in a polypropylene polypropylene 10,000 units glass or syringe22 syringe22 (Orphan 1-2 mL NS22 polypropylene Pharmaceuticals syringe22 International) (F) no preservative22 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 4/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 5. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) PEG-asparaginase (pegasparagase) N/A 750 units/mL23 discard unused IM: maximum volume syringe: 4 h23,24 - cytotoxic3 (pegylated portion23 2 mL; if >2 mL use - discard cloudy asparaginase E. coli) multiple sites23 solution23 750 units/mL - do not shake23 (Enzon) - do not use if (F) stored out of no preservative23 refrigerator for > 48 h23 - do not use if previously frozen23 IV: 100 mL NS or bag: 4 h23,24 D5W23 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 5/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 6. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Azacitidine 100 mg 4 mL SWI25 25 mg/mL25 8 h F, SC syringe25 45 min RT25 - cytotoxic3 (Celgene) 45 min RT25 (RT) shake vigorously25 prepare immediately - discard if contains no preservative25 before use and use large particles25 within 45 min, or discard. - re-suspend syringe contents before injection by vigorously rolling syringe between palms25 BCG 81 mg do not shake; roll to 10.5 ± 8.7×108 2 h F, RT26 50 mL NS26 2 h F or RT after - cytotoxic7 (Sanofi Pasteur) reconstitute26 CFU/vial reconstitution26 (F)(PFL) (Connaught preservative26 3 mL supplied strain)26 **(PFL)26 diluent26 record time of reconstitution BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 6/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 7. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) BCG (Tice substrain) 1 mL preservative 1 to 8×108 2 h F (PFL)27 transfer from vial to 2 h F27 - cytotoxic3 50 mg = 1 to 8 x 108 free NS for CFU/vial27 60 mL syringe, rinse - overfill unknown CFU injection27 vial with another 1 mL - protect from (Hospira/Organon) NS. Add rinse to light27 (F)(PFL) use reconstitution same 60 mL syringe. - do not filter27 no preservative27 device provided qs to 50 mL with NS27 allow to stand for a few minutes, then gently swirl to suspend27 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 7/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 8. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Bendamustine 25 mg single-use vial 25 mg vial: add 5 5 mg/mL28 30 minutes28 500 mL NS28 24 h F, 3 h RT28 - cytotoxic3 100 mg single-use vial mL SWI (Cephalon) 100 mg vial: add 20 0.2-0.6 mg/mL28 (RT)(PFL) mL SWI28 no preservative28 Shake well to yield a clear, colourless to a pale yellow solution. Expected to completely dissolve within 5 minutes28 Bevacizumab 100 mg/4 mL N/A 25 mg/mL29 discard unused 1.4-16.5 mg/mL30 48 h F, RT21,29,30 - cytotoxic7 400 mg/16 mL portion29 - do not shake29 (Roche) 100-250 mL NS (F)(PFL) only29,30 do not shake no preservative29 Bleomycin 15 IU 6 mL*NS31 2.5 IU/mL 48 h F31 50 mL*NS31 24 h RT31 - cytotoxic7 (NB: dose in units only) - no overfill32 (Bristol) (F) no preservative31 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 8/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 9. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Bleomycin 15 IU 6 mL*NS or SWI33 2.5 IU/mL33 48 h F, 24 h RT33 50 mL *NS, SWI33 24 h RT34 - cytotoxic7 (NB: dose in units only) - no overfill35 (Hospira) (F)(PFL) no preservative33 Bortezomib 3.5 mg 3.5 mL NS36 1 mg/mL36 2d RT13,37 syringe36 8 h RT38 - cytotoxic7 (Ortho Biotech formally Millennium) (RT)(PFL) no preservative36 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 9/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 10. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Busulfan 60 mg/10 mL N/A use 5-micron nylon discard unused NS or D5W (dilute in complete - cytotoxic7 (Orphan Medical) filter provided with portion39 volume 10 times the administration within (F) ampoule to busulfan volume to ~ 12 h F: NS39 no preservative39 withdraw drug39 0.5 mg/mL)39 8 h RT: NS, D5W 6 mg/mL39 Carboplatin 50 mg/5 mL N/A 10 mg/mL40 discard unused 0.3-10 mg/mL41 24 h RT,42 48 h F40 - cytotoxic7 150 mg/15 mL portion40 - do NOT use 450 mg/45 mL NS, D5W11,40 aluminum- (Hospira) containing needle, (RT)(PFL) do NOT use syringe or tubing41 no preservative40 aluminum-containing needle or syringe41 Carboplatin 50 mg/5 mL N/A 10 mg/mL43 discard unused 0.5-10 mg/mL44 8 h RT43 - cytotoxic7 150 mg/15 mL portion RT43 - do NOT use 450 mg/45 mL NS, D5W11,43,45 aluminum- (Novopharm) containing needle, (RT)(PFL) do NOT use syringe or tubing43 no preservative43 aluminum-containing needle or syringe43 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 10/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 11. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Carmustine 100 mg 3 mL diluent 3.3 mg/mL in 10% 24 h F, 8 h RT46 glass46 or polyolefin 24 h F: in glass,46 or - cytotoxic7 (Bristol Labs) (supplied)46 ethanol46 container11 polyolefin container11 - do not use if (F) product has oily no preservative46 diluent to reach RT, 500 mL NS or D5W46 use within 4 h of droplets46 then dissolve drug reconstitution RT46 with 3 mL diluent; add 27 mL SWI46 record time of reconstitution Cetuximab 100 mg/50 mL N/A 2 mg/mL47 discard unused syringe47 12 h F, 8 h RT47 - cytotoxic3 200 mg/100 mL portion after 12 h - administer with a (ImClone/BMS) F, 8 h RT47 0.2 or 0.22 micron (F) low protein binding do not dilute sterile evacuated 12 h F, 8 h RT47 in-line filter47 do not shake container or bag e.g. - normal saline no preservative47 polyolefin, may be used to polyethylene, flush the line47 ethylene vinyl - solution may acetate, DEHP contain white plasticized PVC, PVC particulates which bag, or glass47 do not affect product quality47 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 11/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 12. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Cisplatin 10 mg/10 mL N/A 1 mg/mL48 48 h RT49 < 60 mg: 100 mL NS* 48 h RT49 - cytotoxic7 50 mg/50 mL > 60 mg: 250 mL NS* - do NOT use 100 mg/100mL aluminum- (Hospira) 500 or 1000 mL *NS, containing needle, (RT)(PFL) D5-NS, D5-1/2S; D5- syringe or tubing48 no preservative48 NS with mannitol; D5- 1/2S with mannitol48,50; D5W- 1/3S with mannitol48 do NOT use aluminum-containing needle or syringe48 Cladribine 10 mg/10 mL N/A 1 mg/mL51 discard unused SC syringe52 48h F, end of day - cytotoxic7 (Janssen-Ortho) portion51 RT13,51,53,54 - shake vigorously (F)(PFL) cassette51 to dissolve any no preservative51 precipitates from refrigeration11 - bacteriostatic NS contains benzyl alcohol51 500 mL NS only51 24 h RT51 Do NOT use D5W51 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 12/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 13. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) qs to 100 mL with at least 7 days51 bacteriostatic NS only via SIMS DELTEC INC. MEDICATION CASSETTES®51; filter drug and diluent through 0.22u filter as each solution is being introduced into the medication Clodronate 300 mg/10 mL N/A 30 mg/mL discard unused 500 mL NS or D5W55 12 h RT55 - noncytotoxic (Oryx) portion55 (RT) no preservative55 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 13/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 14. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Cyclophosphamide 200 mg NS56 20 mg/mL56 72 h F,56,57 24 h < 1 g: 100 mL NS* 72 h F,56,57 24 h RT56 - cytotoxic7 500 mg RT56 > 1 g: 250 mL NS* 1000 mg 200 mg: 10 mL high dose in BMT: 2000 mg 500 mg: 25 mL may need 500 NS* (Baxter) 1000 mg: 50 mL (RT)(PFL) 2000 mg: 100 mL56 NS, D5W, D5NS56 no preservative56 Cyclosporine N/A 50 mg/mL58 discard unused NS, D5W58 dilute immediately prior - cytotoxic3 50 mg/1 mL portion58 to use58 250 mg/5 mL dilute to concentration (Novartis) between 1:20 and - polyoxyethylated (RT)(PFL) 1:10058 castor oil/ethanol no preservative58 vehicle58 - do NOT refrigerate or freeze58 - use non-PVC bag and tubing59 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 14/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 15. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Cytarabine 100 mg/1 mL N/A 100 mg/mL60 24 h RT60 100 mL*NS, Water for 72 h F, 24 h RT from - cytotoxic7 1000 mg/10mL Injection, D5W, initial vial puncture60 - do not use for IT 2000 mg/20mL record time of Lactated Ringer’s60 injection (Hospira) puncture (RT)(PFL) no preservative60 Cytarabine IT injection60 N/A 100 mg/mL60 24 h RT60 diluents containing use within 4 h of initial - cytotoxic7 100 mg/1 mL preservatives should vial puncture11,13 - auxiliary label62: 1000 mg/10mL record time of NOT be used for “IT” 2000 mg/20mL puncture intrathecal - label to include (Hospira) administration60 route in full (i.e., (RT)(PFL) INTRATHECAL no preservative60 qs to 6 mL with injection) attached preservative free NS61 to both syringe and outer ziplock bag62 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 15/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 16. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Cytarabine SC injection: 100 mg: 5 mL BWI63 100 mg: 48 h RT63,64 syringe 14 d F, 48 h RT64 - cytotoxic7 100 mg 20 mg/mL63 - for high dose (Pfizer) use, do not use (RT)(PFL) diluent containing no preservative63 benzyl alcohol65 - do not use for IT injection Dacarbazine 100 mg 100 mg: 9.9 mL 10 mg/mL66 72 h F, 8 h RT66 250-500 mL*NS or 24 h F, 8 h RT66 - cytotoxic7 200 mg SWI66 D5W - protect container (Abraxis) 200 mg: 19.7 mL **(PFL)11,66 from light during (F)(PFL) SWI66 see Special storage and no preservative66 Precautions/Notes administration67 Column - overfill unknown Dacarbazine 200 mg 200 mg: 19.7 mL 10 mg/mL68 48 h F, 8 h RT68 0.19–3.0 mg/mL13,68 24 h F68 - cytotoxic7 600 mg SWI68 - protect container (Hospira) 600 mg: 59.1 mL (PFL)69 250-500 mL*NS or **(PFL)67 from light during (F)(PFL) SWI68 D5W see Special storage and no preservative68 Precautions/Notes administration67 Column - no overfill69,35 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 16/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 17. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Dactinomycin 0.5 mg 1.1 mL 0.5 mg/mL71 24 h F, RT72 syringe71,73 24 h F, RT 74 - cytotoxic3 (Ovation)70 SWI(preservative (500 mcg/mL) - do not filter71,73 (RT)(PFL) free)71 . no preservative71 Do NOT use SWI with preservative (may form precipitate)71 BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Daunorubicin 20 mg 4 mL SWI75 5 mg/mL75,78 48 h F, 24 h RT77 100-250 mL in 24 h RT, 48 h F75 - cytotoxic3 (Erfa Canada Inc.)75 isotonic solution e.g., (RT)(PFL)76 NS75 no preservative77 no data for D5W77 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 17/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 18. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Daunorubicin 20 mg 4 mL SWI79 5 mg/mL79 24 h RT, 48 h F79 100-250 mL 48 h F, 24 h RT79 - cytotoxic7 (Novopharm) NS or D5W11 (RT)(PFL) (PFL)79 **(PFL)79 no preservative79 Dexrazoxane 250 mg supplied diluent80: 10 mg/mL80 6 h F80 empty viaflex bag80 6 h RT81 - cytotoxic82 500 mg 250 mg: 25 mL (Pfizer) 500 mg: 50 mL (RT) no preservative80 Docetaxel 20 mg/0.5 mL supplied diluent : 10 mg/mL83 48 h F, RT13,85 0.3–0.74 mg/mL complete - cytotoxic7 80 mg/2 mL - if vials were administration within - non-PVC bag and (Sanofi-Aventis) refrigerated, allow to (e.g., 250 mL NS or 4 h F,83 48 h RT86,87 tubing only83 (F, RT) (PFL) warm for 5 min at D5W)83 no preservative83 RT. Withdraw entire contents of the diluent and inject the entire contents of the syringe into the corresponding concentrate vial. Mix by repeated inversions for 45 sec DO NOT SHAKE Let sit for 5 minutes83,84 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 18/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 19. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Doxorubicin 10 mg NS, SWI, D5W88 2 mg/mL88 48 h F, 24 h syringe88 48 h F, 24 h RT13,89 - cytotoxic7 50 mg (NS reconstitution RT13,88 150 mg takes longer) (Hospira) 10 mg: 5 mL (RT)(PFL) 50 mg: 25 mL no preservative88 150 mg: 75 mL 0.2–2 mg/mL90 48 h F, 24 h RT13,90 100 mL*NS88 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 19/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 20. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Doxorubicin 10 mg/5 mL N/A 2 mg/mL 8 h91 syringe91 48 h F, 24 h RT91 - cytotoxic7 20 mg/10 mL from initial vial 50 mg/25 mL record time of puncture 200 mg/100 mL puncture (Novopharm) (F)(PFL) no preservative91 Doxorubicin 10 mg/5 mL N/A 2 mg/mL92 discard unused syringe92 48 h F, 24 h RT92 - cytotoxic3 50 mg/25 mL portion92,74 200 mg/100 mL (Pfizer) (F) no preservative92 Doxorubicin Pegylated Liposomal N/A 2 mg/mL93 discard unused < 90 mg: 250 mL 24 h F93 - cytotoxic7 20 mg/10 mL portion93 D5W only93 - do not filter93 50 mg/25 mL (Schering) ≥ 90 mg: 500mL D5W (F) only no preservative93 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 20/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 21. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Epirubicin 10 mg/5 mL N/A 2 mg/mL94 8 h94 syringe94 48 h F, 24 h RT from - cytotoxic7 50 mg/25 mL initial vial puncture94 200 mg/100 mL record time of (Pfizer) puncture (F)(PFL) no preservative94 100 mL*NS or D5W11 2 d F, RT: NS or D5W49 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 21/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 22. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Etoposide 100 mg/5 mL N/A 20 mg/mL95 14 d RT11,13,96,97,98 0.2– 0.4 mg/mL95 0.2 mg/mL: - cytotoxic7 500 mg/25 mL 48 h RT13,95 - use non-PVC bag 1000 mg/50 mL 500 mL*NS or D5W95 0.4 mg/mL: and tubing only (BMS) 24 h RT95 (RT) preservative95 Etoposide 0.2-0.3 mg/mL: 100 mg/5 mL N/A 20 mg/mL99 discard unused NS 7 d F,100 2 d RT87,100 - cytotoxic7 200 mg/10 mL portion99 0.4-0.5 mg/mL: - use non-PVC bag 500 mg/25 mL Stabilty is 1 d F,100 1 d RT100 and tubing only 1000 mg/50 mL concentration 0.6-9.0mg/mL: (Novopharm) dependent generally unstable100 (RT)(PFL) 9.5 mg/mL: no preservative99 2 d F,100 1d RT100 10-12 mg/mL: 7 d F,100 2 d RT87,100 D5W99 4 h RT99,101 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 22/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 23. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Fludarabine 50 mg 2 mL SWI102 25 mg/mL102 48 h F or RT13,49 dilute to maximum of 48 h F, RT13,49 - cytotoxic7 (Berlex) 1 mg/mL102,103 (F) no preservative102 100 mL*NS or D5W102 Fludarabine 50 mg N/A 25 mg/mL104 discard unused dilute to maximum of 48 h F, 24 h RT104 - cytotoxic7 (Novopharm) portion104 1 mg/mL104 (F) no preservative104 100 mL*NS or D5W BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 23/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 24. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Fluorouracil 5000 mg/100 mL N/A 50 mg/mL105 8 h RT105,106 syringe13 48 h RT13,21,106 - cytotoxic7 (Hospira) (RT)(PFL) no preservative105 2-10 mg/mL in 24 h RT105,106 D5W105,106 50-1000 mL*D5W CIVI: ambulatory complete within pump106 8 d11,13,107 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 24/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 25. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Gemcitabine 200 mg 200 mg: 5 mL NS 38 mg/mL108 48 h RT108,109 syringe108 48 h RT,13,108,109 - cytotoxic7 1000 m g 1000 mg: 25 mL (Eli-Lilly) NS108 (RT) no preservative108 0.1–10 mg/mL 48 h F, RT13,108,109 NS108,109 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 25/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 26. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Gemcitabine 200 mg 200 mg vial: 5 mL 38 mg/mL110 48 RT110,112,74 syringe110 24 h RT110,112 - cytotoxic7 1000 mg NS 2000 mg 1000 mg vial: 25 mL (Hospira) NS RT110 2000 mg vial: 50 mL no preservative111 NS110 26 mg/mL-0.1 mg/mL 48 h RT112,74 NS110,112 Gemcitabine 200 mg 200 mg vial: 5mL 38 mg/mL114 24 h RT114 38 mg/mL – 0.1 24 RT114 - cytotoxic7 1000 mg NS mg/mL NS114 (Novopharm) 1000 mg vial: 25 mL RT NS114 no preservative113 Gemcitabine 200 mg 200 mg vial: 5 mL 38 mg/mL115 48 h RT115,116 syringe115 48 h RT115,117,116 - cytotoxic7 1000 mg NS (Sandoz Standard) 1000 mg vial: 25 mL RT NS115 no preservative115 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 26/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 27. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) 38 mg/mL-0.1 mg/mL 48 h RT13,119 NS or D5W115,118 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 27/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 28. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Idarubicin 5 mg vial under negative 1 mg/mL120 48 h F,120 24 h RT syringe 48 h F,13, 120 24 h RT13 - cytotoxic7 10 mg pressure120 (PFL)120 (PFL)120 (Pfizer) (RT)(PFL) 5 mg: 5 mL SWI no preservative120 10 mg: 10 mL SWI120 Idarubicin 5 mg/5 mL N/A 1 mg/mL120 same as syringe Discard 48 h F,13,120 24 - cytotoxic7 10 mg/10 mL reconstituted h RT13 20 mg/20 mL solution121 (PFL)120 (Pfizer) (F)(PFL) no preservative120 Ifosfamide 1000 m g 1000 mg: 20 mL 50 mg/mL122 48 h F13,122 0.6–20 mg/mL122 72 h F122 - cytotoxic7 3000 mg SWI122 24 h F, RT when mixed (Baxter) 3000 mg: 60 mL 500–1000 mL*NS, with mesna11 (RT) 11 SWI D5W, D5-NS, no preservative122 D5-1/2NS, Lactated D5W or Lactated shake well Ringer’s11,122 Ringer’s when mixed Interferon Alfa -2b 18 million IU/3 mL N/A 6 million IU/mL123 48 h F13,123 syringe123 2 d F13,124 - cytotoxic7 (Schering) (F)(or up to 7 days at RT before use) no preservative123 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 28/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 29. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) ≥ 0.3 million IU/mL123 24 h F, RT124 50 mL NS123 Interferon Alfa -2b 25 million IU/2.5 mL N/A 10 million IU/mL123 48 h F13,123 syringe123 2 d F13,124 - cytotoxic7 (Schering) (F)(or up to 7 days at RT before use) no preservative123 ≥ 0.3 million IU/mL123 24 h F, RT124 50 mL NS123 Interferon Alfa -2b 10 million IU 1 mL supplied 10 million IU/mL123 24 h F123 syringe123 24 h F, RT124 - cytotoxic7 (Schering) diluent (SWI) 123 (F) no preservative123 do not shake; roll to reconstitute123 > 0.1 million IU/mL124 48 h RT11,13 100 mL NS125 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 29/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 30. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) 1 mL BWI123 48 h F, RT13,123 syringe125 14 d F, 48 h RT13,125 do not shake; roll to reconstitute123 100 mL NS125 48 h RT11,13 Interferon Alfa -2b 18 million IU 1 mL supplied 18 million IU/mL123 24 h F123 syringe123 24 h F, RT124 - cytotoxic7 (Schering) diluent123 (F) no preservative123 do not shake; roll to reconstitute123 > 0.1 million IU/mL126 48 h RT11,13 100 mL NS125 1 mL BWI123 48 h F, RT13 syringe123 14 d F13,125 do not shake; roll to reconstitute123 100 mL NS125 48 h RT11,13 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 30/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 31. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Irinotecan 40 mg/2 mL N/A 20 mg/mL127 2 days RT13,128,129 0.12– 2.8 mg/mL127 24 h RT: D5W, NS127 - cytotoxic7 100 mg/5 mL - do NOT 500 mg/25 mL 500 mL11 D5W 48 h F: D5W refrigerate if in (Hospira) (preferred), NS127 (PFL)127 NS130 (RT)(PFL) no preservative127 Irinotecan 40 mg/2 mL N/A 20 mg/mL130 discard unused 0.12– 2.8 mg/mL130 24 h RT: D5W, NS130 - cytotoxic7 100 mg/5 mL portion130 - do NOT (Pfizer) 500 mL11 D5W 48 h F: D5W refrigerate if in (RT)(PFL) (preferred), NS130 (PFL)130 NS130 no preservative130 Irinotecan 40 mg/2 mL N/A 20 mg/mL131 discard unused 0.12-2.8 mg/mL131 24 h RT: D5W NS131 - cytotoxic3 100 mg/5 mL portion131,74 48 h F: D5W131 (Sandoz) D5W (recommended) (RT)(PFL) NS131 (PFL)131 no preservative131 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 31/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 32. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Ixabepilone 15 mg 8 mL supplied 2 mg/mL 1 h RT132 0.2 – 0.6 mg/mL in 6 h RT132 - cytotoxic3 (contains 16 mg) diluent Lactated Ringer’s - use 0.2-1.2 45 mg Injection USP (use micron in-line (contains 47 mg) 23.5 mL supplied 2 mg/mL non-PVC infusion filter132 (BMS) diluent container)132 - use non-PVC (F)(PFL) (i.e., DEHP-free) no preservative132 administration set132 Leucovorin 50 mg/5 mL N/A 10 mg/mL133 5 mL vial: discard syringe134 7 d F134 - noncytotoxic 500 mg/50 mL unused portion133 48 h RT87,134 (Hospira) (F)(PFL) 50 mL vial: 8 h no preservative133 0.05-10 mg/mL133 24 h RT133: NS, D5W, Lactated Ringer’s, Ringer’s 50-250 mL* NS, 8 h RT133: D10W, D5- D5W, Lactated NS Ringer’s, Ringer’s, D10W, D5-NS BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 32/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 33. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Leucovorin 50 mg/5 mL N/A135 10 mg/mL135 5 mL vial: discard syringe 8 h135,13 - noncytotoxic 500 mg/50 mL unused portion135 (Novopharm) (F)(PFL)135 50 mL vial: discard unused portion135 0.060-1.0 mg/mL135 24 h RT NS, Lactated Ringer’s, Ringer’s135 50-250 mL* NS, 12 h RT D5W, D5W, Lactated D10W135 Ringer’s, Ringer’s, D10W, D10NS135 6 h RT D5NS135 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 33/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 34. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Mechlorethamine 10 mg do NOT use if 1 mg/mL136 use within 4 h of syringe136 complete - cytotoxic7 (Ovation discoloured or water reconstitution administration Pharmaceuticals/Merck) droplets form in vial RT11,13 4 h of reconstitution no preservative136 before RT11,13,136 reconstitution136 10 mL SWI or NS136 record time of reconstitution 100 mL NS49,136 complete administration within 4 h of reconstitution RT13,49,136 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 34/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 35. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Medroxyprogesterone 250 mg/5 mL N/A 50 mg/mL18 use within 4 h of syringe: IM only18 use within 4 h of initial - cytotoxic7 (Pfizer) initial vial puncture11,137 - auxiliary label: (RT) record time of puncture11,137 shake before use18 preservative18 puncture Medroxyprogesterone Depo N/A 150 mg/mL18 use within 4 h of syringe: IM only18 use within 4 h of initial - cytotoxic7 150 mg/1mL initial puncture11,137 - auxiliary label: (Pfizer) record time of puncture11,137 shake before use18 (RT) puncture preservative18 Melphalan 50 mg 10mL supplied 5 mg/mL138 2 h RT138 0.1– 0.45 mg/mL in complete - cytotoxic7 (GSK) diluent138 NS only138 administration within (RT)(PFL) do NOT 60 min from time of no preservative138 immediately after refrigerate (e.g., > 45 mg and < initial reconstitution at adding diluent, 110 mg in 250 mL RT11 shake vigorously138 NS)* record time of reconstitution BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 35/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 36. Mesna 1000 mg/10mL N/A 100 mg/mL139 14 d F, RT13,139 > 1mg/mL139 48 h F, 24 h RT139 - noncytotoxic (PPC) (RT) NS or D5W preservative139 Methotrexate 50 mg/2mL N/A 25 mg/mL140 50mg: discard syringe 2 d F, RT11,141,142 - cytotoxic7 500 mg/20mL unused portion140 - for high-dose 1 g/40mL regimens (e.g., 1-8 5 g/200mL 500mg, 1 g, 5 g: g/m2 as a single (Hospira) 8 h F, RT140 dose)143 use (RT)(PFL) preservative-free no preservative140 methotrexate11 - do not use for IT injection 0.4–2 mg/mL140 24 h RT140 100 mL* NS, D5W high dose (e.g., 1-8 g/m2 as a single dose)143,144-146: 500– 1000 mL* BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 36/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 37. Methotrexate IT Injection140: N/A 25 mg/mL140 discard unused qs to 6 mL with use within 4 h of initial - cytotoxic7 Only preservative free portion140 preservative free NS61 puncture11,13 - auxiliary label62: methotrexate may be “IT” administered by the - label to include intrathecal route140 route in full (i.e., 50 mg/2mL147 INTRATHECAL (Hospira) injection) attached (RT)(PFL) to both syringe and no preservative140 outer ziplock bag62 Methotrexate 50 mg/2mL N/A 25 mg/mL140 24 h F141 syringe 14 d F87,141 - cytotoxic7 500 mg/20mL - high-dose (Hospira) regimen(e.g., 1-8 (RT)(PFL) g/m2 as a single preservative140 dose)144-146: use preservative-free methotrexate11 - do not use for IT injection 0.4–2 mg/mL140 4 h RT140 e.g., 100 mL*NS, D5W140 Mitomycin 5 mg SWI 0.5 mg/mL148 48 h F, RT13,148 syringe13 14 d F, 48 h RT13,148 - cytotoxic7 20 mg 5 mg: 10 mL (PFL)148 (Novopharm) 20 mg: 40 mL (RT)(PFL) no preservative148 shake well148 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 37/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 38. 0.02-0.04 mg/mL148 3 h RT: D5W 12 h RT: NS NS, D5W, sodium 24 h RT: sodium lactate148 lactate148 Mitomycin 5 mg SWI 0.5 mg/mL149 48 h F, RT13,149 syringe11 14 d F, 48 h RT11,21 - cytotoxic7 20 mg 5 mg: 10 mL (BMS) 20 mg: 40 mL (PFL)149 (RT)(PFL) no preservative149 shake well149 0.02–0.04 mg/mL 12 h RT: NS 3h: D5W NS, D5W, sodium 24 h: sodium lactate149 lactate149 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 38/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 39. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Mitoxantrone 20 mg/10 mL N/A 2 mg/mL150 discard unused 0.2-0.6 mg/mL150 NS: 24 h F, RT150 - cytotoxic7 25 mg/12.5 mL portion150 (Hospira) NS, D5W150 **(PFL)150 (RT)(PFL) no preservative150 > 50 mL*150 Mitoxantrone 20 mg/10 mL N/A 2 mg/mL151 discard unused NS, D5W151 24 h RT151 - cytotoxic7 (Novopharm) portion151 (RT)(PFL) > 50 mL*151 **(PFL)152 no preservative151 Mitoxantrone 20 mg/10 mL N/A 2 mg/mL153 discard unused NS, D5W2 24 h RT153 - cytotoxic7 (Pharmaceutical portion153 > 50 mL*153 Partners of Canada) (RT) no preservative153 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 39/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 40. Octreotide 50 mcg/mL; 100 N/A 50 mcg/mL discard unused sc syringe154 single use vials: use - noncytotoxic mcg/mL; 500 mcg/mL 100 mcg/mL portion154 within 4 h (Novopharm) 500 mcg/mL154 multidose vials: use (F)(PFL) within 14 d F74,154 no preservative multidose vials (5mL): 200 µg/mL (F)(PFL) 154 preservative 200 mcg/mL154 14 d F74,154 infusion: NS154 single use vials or multidose vials: 24 h RT154 Octreotide (Sandostatin) N/A 200 mcg/mL18 discard unused 50–200 mL NS18,11,156 24 h RT18 - noncytotoxic10 1000 mcg/5 mL portion155 (Novartis) (F)(PFL) SC infusion: adjust preservative18 volume to ensure infusion rate of 25 mcg/h18 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 40/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 41. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Octreotide (Sandostatin) N/A 50 mcg/mL discard unused 50-100 mL11,156 24 h RT18 - noncytotoxic10 50 mcg/1 mL 100 mcg/mL portion18 100 mcg/1 mL 500 mcg/mL18 NS18 500 mcg/1 mL (Novartis) SC infusion: adjust (F)(PFL) volume to ensure no preservative18 infusion rate of 25 mcg/h18 Octreotide (Sandostatin LAR) 2 mL supplied 10 mg: 5 mg/mL discard unused deep intragluteal use within 4 h of initial - noncytotoxic10 10 mg diluent 20 mg: 10 mg/mL portion18 administration only18 reconstitution18,13 - do NOT shake 20 mg 30 mg: 15 mg/mL18 30 mg gently run 2 mL (Novartis) down sides of the (F)(PFL) vial; do NOT preservative155 disturb for 2–5 min, then swirl moderately18 record time of reconstitution BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 41/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 42. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Oxaliplatin 50 mg/10 mL N/A 5 mg/mL157 discard unused 0.2-1.3 mg/mL158 0.2-1.3 mg/mL: - cytotoxic7 100 mg/20 mL portion157 14 d F, 48 h RT159,87,158 - do NOT use 200 mg/40 mL 1.3–2 mg/mL157,158 aluminum- (Sanofi-Aventis) 1.3-2 mg/mL: containing needle, (RT)(PFL) 250–500 mL D5W157 48 h F, 24 h RT157 syringe or no preservative157 tubing157 do NOT use NS or other chloride- containing solution157 do NOT use aluminum-containing needle and syringe157 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 42/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
- 43. BC CANCER AGENCY CHEMOTHERAPY PREPARATION AND STABILITY CHART DRUG & STRENGTH Reconstitute To Give: Vial Product Product Stability Special (Storage Prior to Use, With: Stability Precautions/Notes Manufacturer, Preservative Status) Oxaliplatin 50 mg SWI, D5W: 5 mg/mL160 24 h F160 500 mL D5W 24 h F, 6 h RT160 - cytotoxic7 100 mg 50 mg: 10 mL - do NOT use (Sigmacon) 100 mg: 20 mL do NOT use NS or aluminum- (RT) other chloride- containing needle, no preservative160 do NOT use NS or containing solutions syringe or tubing160 other chloride- (degrades)160 containing solution160 do NOT use aluminum-containing do NOT use needle and syringe160 aluminum- containing needle and syringe160 Paclitaxel 30 mg/5 mL N/A 6 mg/mL162 8 h RT162 0.3–1.2 mg/mL in NS, 0.3-1.2 mg/mL: - cytotoxic7 100 mg/16.7 mL D5W162 24 h RT162 - use non-PVC bag 300 mg/50 mL (e.g., 100–1000 mL)* and tubing with in- (Biolyse) 0.1 mg/mL: line filter162 (RT)161 44 h F, RT163 no preservative 0.1 mg/mL in NS163 0.012-0.09 mg/mL: 0.012-0.1 mg/mL164 16 h RT164 BC Cancer Agency Chemotherapy Preparation and Stability Chart© version 2.00 43/77 Activation Date: 2 March 2006 Revised Date: 1 July 2010
