Chemistry 1 Revision Cards
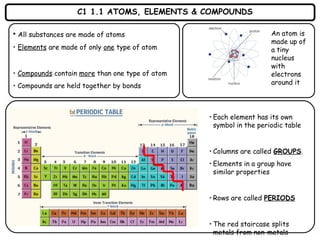
- 1. C1 1.1 ATOMS, ELEMENTS & COMPOUNDS • All substances are made of atoms • Elements are made of only one type of atom • Compounds contain more than one type of atom • Compounds are held together by bonds • Each element has its own symbol in the periodic table • Columns are called GROUPS. • Elements in a group have similar properties • Rows are called PERIODS • The red staircase splits metals from non-metals An atom is made up of a tiny nucleus with electrons around it
- 2. C1 1.2 ATOMIC STRUCTURE • Atoms contain PROTONS, NEUTRONS & ELECTRONS • Protons and Neutrons are found in the NUCLEUS • Electrons orbit the nucleus • ATOMIC NUMBER the number of protons in the nucleus the periodic table is arranged in this order • MASS NUMBER the number of protons plus neutrons Number of neutrons = Mass Number – Atomic Number Any atom contains equal numbers of protons and electrons PARTICLE RELATIVE CHARGE RELATIVE MASS Proton +1 (positive) 1 Neutron 0 (neutral) 1 Electron -1 (negative) 0
- 3. C1 1.3 ELECTRON ARRANGEMENT • Electrons are arranged around the nucleus in SHELLS (or energy levels) • The shell closest to the nucleus has the lowest energy • Electrons occupy the lowest available energy level • Atoms with the same number of electrons in the outer shell belong to the same GROUP in the periodic table • Number of outer electrons determine the way an element reacts • Atoms of the last group (noble gases) have stable arrangements and are unreactive This is how we draw atoms and their electrons Low energy shell High energy shell Sodium
- 4. C1 1.4 FORMING BONDS • Atoms can react to form compounds in a number of ways: i) Transferring electrons IONIC BONDING ii) Sharing electrons COVALENT BONDING IONIC BONDING • When a metal and non-metal react • Metals form positive ions • Non-metals from negative ions • Opposite charges attract • A giant lattice is formed COVALENT BONDING • When 2 non-metals bond • Outermost electrons are shared • A pair of shared electrons forms a bond CHEMICAL FORMULAE • Tells us the ratio of each element in the compound • In ionic compounds the charges must cancel out: E.g. MgCl2 We have 2 chloride ions for every magnesium ion
- 5. H2 + O2 H2O Add a 2 to the products side to make the oxygen balance H2 + O2 2H2O This has changed the number of hydrogen atoms so we must now adjust the reactant side: 2H2 + O2 2H2O C1 1.5 CHEMICAL EQUATIONS • Chemical equations show the reactants (what we start with) and the products (what we end up with) • We often use symbol equations to make life easier CaCO3 CaO + CO2 MAKING EQUATIONS BALANCE Equations MUST balance We can ONLY add BIG numbers to the front of a substance We can tell elements within a compound by BIG letters CaCO3 this is a compound made of 3 elements (calcium, carbon and oxygen) Ca = 1 C = 1 O = 3 Ca = 1 C = 1 O = 3 • This is balanced – same number of each type of atom on both sides of the equation • We can check this by counting the number of each type on either side H = 2 O = 2 H = 2 O = 1 H = 2 O = 2 H = 4 O = 2
- 6. C1 2.1 LIMESTONE & ITS USES • Limestone is made mainly of Calcium Carbonate • Calcium carbonate has the chemical formulae CaCO3 • Some types of limestone (e.g. chalk) were formed from the remains of animals and plants that live millions of years ago USE IN BUILDING We use limestone in many buildings by cutting it into blocks. Other ways limestone is used: Cement = powdered limestone + powdered clay Concrete = Cement + Sand + Water HEATING LIMESTONE Breaking down a chemical by heating is called THERMAL DECOMPOSITION Calcium Calcium + Carbon Carbonate Oxide Dioxide CaCO3 CaO + CO2 ROTARY LIME KILN This is the furnace used to heat lots of calcium carbonate and turn it into calcium oxide Calcium oxide is used in the building and agricultural industries
- 7. C1 2.2 REACTIONS OF CARBONATES • Buildings made from limestone suffer from damage by acid rain • This is because carbonates react with acid to form a salt, water and carbon dioxide Calcium + Hydrochloric Calcium + Water + Carbon Carbonate Acid Chloride Dioxide CaCO3 + 2HCl CaCl2 + H2O + CO2 TESTING FOR CO2 • We use limewater to test for CO2 • Limewater turns cloudy • A precipitate (tiny solid particles) of calcium carbonate forms causing the cloudiness! HEATING CARBONATES Metal carbonates decompose on heating to form the metal oxide and carbon dioxide MgCO3 MgO + CO2
- 8. C1 2.3 THE LIMESTONE REACTION CYCLE • Limestone is used widely as a building material • We can also use it to make other materials for the construction industry Calcium Carbonate + Heat Calcium Oxide Calcium Oxide + Water Calcium Hydroxide (Limewater) Calcium Carbonate Calcium Oxide Calcium Hydroxide Calcium Hydroxide Solution Step 1: Add Heat CaCO3 CaO + CO2 Step 2: Add a bit of water CaO + H2O Ca(OH)2 Step 3: Add more water & filter Ca(OH0)2 + H2O Ca(OH)2 (aq) Step 4: Add CO2 Ca(OH)2 + CO2 CaCO3 + H2O Limestone
- 9. C1 2.4 CEMENT & CONCRETE CEMENT Made by heating limestone with clay in a kiln MORTAR Made by mixing cement and sand with water CONCRETE Made by mixing crushed rocks or stones (called aggregate), cement and sand with water C1 2.5 LIMESTONE ISSUES BENEFITS • Provide jobs • Lead to improved roads • Filled in to make fishing lakes or for planting trees • Can be used as landfill sites when finished with DRAWBACKS • Destroys habitats • Increased emissions • Noisy & Dusty • Dangerous areas for children • Busier roads • Ugly looking
- 10. C1 3.1 EXTRACTING METALS • A metal compound within a rock is called an ORE • The metal is often combined with oxygen • Ores are mined from the ground and then purified Whether it’s worth extracting a particular metal depends on: How easy it is to extract How much metal the ore contains The reactivity series helps us decide the best way to extract a metal: Metals below carbon in the series can be reduced by carbon to give the metal element Metals more reactive than carbon cannot be extracted using carbon. Instead other methods like ELECTROLYSIS must be used THE REACTIVITY SERIES
- 11. C1 3.2 IRON & STEELS • Iron Ore contains iron combined with oxygen • We use a blast furnace and carbon to extract it (as it’s less reactive than carbon) • Carbon REDUCES the iron oxide; Iron (III) Oxide + Carbon Iron + Carbon Dioxide • Iron from the blast furnace contains impurities: Makes it hard and brittle Can be run into moulds to form cast iron Used in stoves & man-hole covers • Removing all the carbon impurities gives us pure iron Soft and easily shaped Too soft for most uses Need to combine it with other elements • A metal mixed with other elements is called an ALLOY E.g. Steel Iron with carbon and/or other elements There are a number of types of steel alloys: Carbon steels Low-alloy steels High-alloy steels Stainless steels
- 12. C1 3.3 ALUMINIUM & TITANIUM Aluminium Titanium Property • Shiny • Light • Low density • Conducts electricity and energy • Malleable – easily shaped • Ductile – drawn into cables and wires • Strong • Resistant to corrosion • High melting point – so can be used at high temperatures • Less dense than most metals Use • Drinks cans • Cooking foil • Saucepans • High-voltage electricity cables • Bicycles • Aeroplanes and space vehicles • High-performance aircraft • Racing bikes • Jet engines • Parts of nuclear reactors • Replacement hip joints Extraction Electrolysis • Aluminium ore is mined and extracted. • Alumminium oxide (the ore) is melted • Electric current passed through at high temperature Expensive process – need lots of heat and Displacement & Electrolysis • Use sodium or potassium to displace titanium from its ore • Get sodium and magnesium from electrolysis Expensive – lots of steps involved, &
- 13. C1 3.4 EXTRACTING COPPER COPPER-RICH ORES These contain lots of copper. There are 2 ways to consider: 1. Smelting • 80% of copper is produced this way • Heat copper ore strongly in a furnace with air Copper + Oxygen Copper + Sulphur Sulphide Dioxide • Then use electrolysis to purify the copper • Expensive as needs lots of heat and electricity 2. Copper Sulphate • Add sulphuric acid to a copper ore • Produces copper sulphate • Extract copper using electrolysis or displacement LOW GRADE COPPER ORES These contain smaller amount of copper. There are 2 main ways: 1. Phytomining • Plants absorb copper ions from low-grade ore • Plants are burned • Copper ions dissolved by adding sulphuric acid • Use displacement or electrolysis to extract pure copper 2. Bioleaching • Bacteria feed on low-grade ore • These produce a waste product that contains copper ions • Use displacement or electrolysis to extract pure copper
- 14. C1 3.5 USEFUL METALS TRANSITION METALS • Found in the central block of the periodic table Properties: • Good conductors of electricity and energy • Strong • Malleable – easily bent into shape Uses: • Buildings • Transport (cars, trains etc) • Heating systems • Electrical wiring Example: Copper 1. Water pipes – easily bent into shape, strong, doesn’t react with water 2. Wires – ductile and conduct electricity COPPER ALLOYS Bronze – Copper + Tin - Tough - Resistant to corrosion Brass – Copper + Zinc - Harder but workable ALUMINIUM ALLOYS • Alloyed with a wide range of other elements • All have very different properties • E.g. in aircraft or armour plating! GOLD ALLOYS • Usually add Copper to make jewellery last longer
- 15. C1 3.6 METALLIC ISSUES EXPLOITING ORES Mining has many environmental consequences: • Scar the landscape • Noisy & Dusty • Destroy animal habitats • Large heaps of waste rock • Make groundwater acidic • Release gases that cause acid rain RECYCLING METALS • Recycling aluminium saves 95% of the energy normally used to extract it! • This saves money! • Iron and steel are easily recycled. As they are magnetic they are easily separated • Copper can be recycled too – but it’s trickier as it’s often alloyed with other elements BUILDING WITH METALS Benefits • Steel is strong for girders • Aluminium is corrosion resistant • Many are malleable • Copper is a good conductor and not reactive Drawbacks • Iron & steel can rust • Extraction causes pollution • Metals are more expensive than other materials like concrete
- 16. C1 4.1 FUELS FROM CRUDE OIL CRUDE OIL • A mixture of lots of different compounds [A mixture is 2 or more elements or compounds that are not chemically bonded together] • We separate it into substances with similar boiling points • These are called fractions • This is done in a process called fractional distillation HYDROCARBONS Nearly all the compounds in crude oil are hydrocarbons Most of these are saturated hydrocarbons called alkanes Methane CH4 Ethane C2H6 Propane C3H8 Butane C4H10 General formula for an alkane is CnH(2n+2)
- 17. C1 4.2 FRACTIONAL DISTILLATION This is the process by which crude oil is separated into fractions These are compounds with similar sized chains Process relies on the boiling points of these compounds The properties a fraction has depend on the size of their hydrocarbon chains SHORT CHAINS ARE: Very flammable Have low boiling points Highly volatile (tend to turn into gases) Have low viscosity (they flow easily) Long chains have the opposite of these! Crude oil fed in at the bottom Temperature decreases up the column Hydrocarbons with smaller chains found nearer the top
- 18. C1 4.3 BURNING FUELS COMPLETE COMBUSTION Lighter fractions from crude oil make good fuels They release energy when they are oxidised burnt in oxygen: propane + oxygen carbon dioxide + water POLLUTION Fossil fuels also produce a number of impurities when they are burnt These have negative effects on the environment The main pollutants are summarised below Sulphur Dioxide • Poisonous gas • It’s acidic • Causes acid rain • Causes engine corrosion Carbon Monoxide • Produced when not enough oxygen • Poisonous gas • Prevents your blood carrying oxygen around your body Nitrogen Oxide • Poisonous • Trigger asthma attacks • Can cause acid rain Particulates • Tiny solid particles • Contain carbon and unburnt hydrocarbon • Carried in the air • Damage cells in our lungs • Cause cancer
- 19. C1 4.4 CLEANER FUELS Burning fuels releases pollutants that spread throughout the atmosphere: CATALYTIC CONVERTERS • Reduces the carbon monoxide and nitrogen oxide produced • They are expensive • They don’t reduce the amount of CO2 GLOBAL DIMMING • Caused by particulates • Reflect sunlight back into space • Not as much light gets through to the Earth CARBON MONOXIDE Formed by incomplete combustion GLOBAL WARMING • Caused by carbon dioxide • Causing the average global temperature to increase SULPHUR DIOXIDE • Caused by impurities in the fuel • Affect asthma sufferers • Cause acid rain damages plants & buildings Carbon + Nitrogen Carbon + Nitrogen Monoxide Oxide Dioxide
- 20. C1 4.5 ALTERNATIVE FUELS These are renewable fuels sources of energy that could replace fossil fuels (coal, oil & gas) BIODIESEL ETHANOL HYDROGEN + • Less harmful to animals • Breaks down 5 × quicker • Reduces particulates • Making it produces other useful products •‘CO2 neutral’ – plants grown to create it absorb the same amount of CO2 generated when it’s burnt • Easily made by fermenting sugar cane • Gives off CO2 but the sugar cane it comes from absorbs CO2 when growing • Very clean – no CO2 • Water is the only product - • Large areas of farmland required • Less food produced Famine • Destruction of habitats • Freezes at low temps • Large areas of farmland required • Less food produced as people use it for fuel instead! • Hydrogen is explosive • Takes up a large volume storage becomes an issue
- 21. C1 5.1 CRACKING HYDROCARBONS CRACKING Breaking down large hydrocarbon chains into smaller, more useful ones SATURATED OR UNSATURATED? We can react products with bromine water to test for saturation: Positive Test: Unsaturated + Bromine COLOURLESS hydrocarbon Water = ALKENES Negative Test: Saturated + Bromine NO RECTION Hydrocarbon Water (orange) = ALKANES CRACKING PROCESS 1. Heat hydrocarbons to a high temp; then either: 2. Mix them with steam; OR 3. Pass the over a hot catalyst EXAMPLE OF CRACKING Cracking is a thermal decomposition reaction: C10H22 C5H12 + C3H6 + C2H4 ALKENES • These are unsaturated hydrocarbons • They contain a double bond • Have the general formula CnH2n Decane Pentane Propene Ethene 800o C
- 22. C1 5.2 POLYMERS FROM ALKENES PLASTICS Are made from lots of monomers joined together to make a polymer HOW DO MONOMERS JOIN TOGETHER? • Double bond between carbons ‘opens up’ • Replaced by single bonds as thousands of monomers join up • It is called POLYMERISATION MONOMERS POLYMER Ethene Poly(ethene) n Simplified way of writing it: ‘n’ represent a large repeating number
- 23. C1 5.3 NEW & USEFUL POLYMERS DESIGNER POLYMER Polymer made to do a specific job Examples of uses for them: • Dental fillings • Linings for false teeth • Packaging material • Implants that release drugs slowly Light-Sensitive Plasters • Top layer of plaster peeled back • Lower layer now exposed to light • Adhesive loses stickiness • Peels easily off the skin SMART POLYMERS Have their properties changed by light, temperature or other changes in their surroundings Hydrogels • Have cross-linking chains • Makes a matrix that traps water • Act as wound dressings • Let body heal in moist, sterile conditions • Good for burns Shape memory polymers • Wound is stitched loosely • Temperature of the body makes the thread tighten • Closes the wound up with the right amount of force
- 24. C1 5.4 PLASTIC WASTE NON-BIODEGRADABLE • Don’t break down • Litter the streets and shores • Harm wildlife RECYCLING • Sort plastics into different types • Melted down and made into new products • Saves energy and resources…BUT • Hard to transport and • Need to be sorted into specific types DISADVANTAGES OF BIODEGRADABLE PLASTICS • Farmers sell crops like corn to make plastics • Demand for food goes up • Food prices go up less can afford it STARVATION • Animal habitats destroyed to make new farmland • Unsightly • Last 100’s of years • Fill up landfill sites BIODEGRADABLE PLASTICS • Plastics that break down easily • Granules of cornstarch are built into the plastic • Microorganisms in soil feed on cornstarch • This breaks the plastic down
- 25. C1 5.5 ETHANOL There are 2 main ways to make ethanol 2) ETHENE Hydration reaction water is added Ethene + Steam Ethanol C2H4 + H2O C2H5OH + Continuous process – lots made! + Produces no waste products - Requires lots of heat and energy - Relies on a non-renewable resource 1) FERMENTATION Sugar from plants is broken down by enzymes in yeast Sugar + Yeast Ethanol + Carbon Dioxide 80% of ethanol is made this way + Uses renewable resources -Takes longer to produce - CO2 is given off A molecule of ethanol HH-C-C-O H H H H USES FOR ETHANOL • Alcohol • Perfume • Rocket Fuel • Solvents • Antiseptic wipes
- 26. C1 6.1 EXTRACTING VEGETABLE OIL There are 2 ways to extract vegetable oils from plants: 2) DISTILLATION 1. Plants are put into water and boiled 2. Oil and water evaporate together 3. Oil is collected by condensing (cooling the gas vapours) Lavender oil is one oil extracted this way 1) PRESSING 1. Farmers collect seeds from plants 2. Seeds are crushed and pressed 3. This extracts oil from them 4. Impurities are removed 5. Oil is processed to make it into a useful product FOOD AND FUEL Vegetable oils are important foods: • Provide important nutrients (e.g. vitamin E) • Contain lots of energy so can also be used as fuels • Unsaturated oils contain double bonds (C=C) they decolourise Bromine water Food Energy (kJ) Veg Oil 3900 Sugar 1700 Meat 1100 Table for info only – don’t memorise it!
- 27. C1 6.2 COOKING WITH VEGETABLE OILS COOKING IN OIL • Food cooks quicker • Outside becomes crispier • Inside becomes softer • Food absorbs some of the oil • Higher energy content • Too much is unhealthy HARDENING VEGETABLE OILS • Reacting vegetable oils with HYDROGEN hardens them increases melting points • Makes them solid at room temperature makes them into spreads! • Double bonds converted to single bonds C=C C-C • Now called a HYDROGENATED OIL • Reaction occurs at 60o C with a nickel catalyst + 60o C + Nickel catalyst Double bonds converted to single bonds Margarine
- 28. C1 6.3 EVERYDAY EMULSIONS Oils do not dissolve in water Emulsion Where oil and water are dispersed (spread out) in each other These often have special properties EMULSION EXAMPLES 1. Mayonnaise 2. Milk 3. Ice cream 4. Cosmetics – face cream, lipstick etc 5. Paint EMULSIFIERS • Stop water and oil separating out into layers • Emulsifiers have 2 parts that make them work: 1.Hydrophobic tail – is attracted to oil 2.Hydrophilic head – is attracted to water. It has a negative charge Oil droplet Emulsifier molecule Water -
- 29. C1 6.4 FOOD ISSUES E NUMBER Additives approved for use in Europe EMULSIFIERS • Improve texture and taste of foods containing fats and oils • Makes them more palatable (tasty) and tempting to eat! FOOD ADDITIVES Substance added to food to: • Preserve it • Improve its taste • Improve its texture • Improve its appearance VEG OILS Unsaturated Fats: • Source of nutrients like vitamin E • Keep arteries clear • Reduce heart disease • Lower cholesterol levels ANIMAL FATS Saturated Fats: • Are not good for us • Increase risk of heart disease • Increase cholesterol E.g. chocolate!
- 30. C1 7.1 STRUCTURE OF THE EARTH Atmosphere: Most lies within 10km of the surface Rest is within 100km but it’s hard to judge! Crust: Solid 6km beneath oceans 35km beneath land Core: Made of nickel and iron Outer core is liquid Inner core is solid Radius is 3500km Mantle Behaves like a solid Can flow very slowly Is about 3000km deep!
- 31. C1 7.2 THE RESTLESS EARTH MOVING CONTINENTS The Earth’s crust and upper mantle are cracked into a number of pieces TECTONIC PLATES These are constantly moving - just very slowly Motion is caused by CONVECTION CURRENTS in the mantle, due to radioactive decay PANGAEA If you look at the continents they roughly fit together Scientists think they were once one large land mass called pangaea, which then broke off into smaller chunks PLATE BOUNDARIES Earthquakes and volcanoes happen when tectonic plates meet These are very difficult to predict
- 32. C1 7.3 THE EARTH’S ATMOSPHERE IN THE PAST PHASE 1:PHASE 1: Volcanoes = Steam & CO2 • Volcanoes kept eruptingVolcanoes kept erupting giving outgiving out Steam andand CO2 • The early atmosphere wasThe early atmosphere was nearly all COnearly all CO22 • TheThe earth cooledearth cooled andand water vapourwater vapour condensedcondensed to form theto form the oceansoceans Like this for a billion years! PHASE 2:PHASE 2: Green Plants, Bacteria & Algae = Oxygen • Green plants, bacteriaGreen plants, bacteria and algae ran riot in theand algae ran riot in the oceans!oceans! • Green plants steadilysteadily converted CO2 into O2 by the process ofby the process of photosynthesis • Nitrogen released byreleased by denitrifying bacteria • Plants colonise the land. Oxygen levels steadily increase PHASE 3:PHASE 3: Ozone Layer = Animals & Us • TheThe build up of Obuild up of O22 killed off earlykilled off early organisms - allowingorganisms - allowing evolution of complexevolution of complex organismsorganisms • TheThe O2 created thecreated the Ozone layer (O3) whichwhich blocks harmfulblocks harmful UV rays from the sunfrom the sun • Virtually no COVirtually no CO22 leftleft
- 33. C1 7.4 LIFE ON EARTH No one can be sure how life on Earth first started. There are many different theories: MILLER-UREY EXPERIMENT • Compounds for life on Earth came from reactions involving hydrocarbons (e.g. methane) and ammonia • The energy for this could have been provided by lightning OTHER THEORIES 1. Molecules for life (amino acids) came on meteorites from out of space 2. Actual living organisms themselves arrived on meteorites 3. Biological molecules were released from deep ocean vents The experiment completed by Miller and Urey
- 34. C1 7.5 GASES IN THE ATMOSPHERE THE ATMOSPHERE TODAY: The main gases in the atmosphere today are: 1. Nitrogen 78% 2. Oxygen 21% 3. Argon 0.9% 4. Carbon Dioxide 0.04% CARBON DIOXIDE: • Taken in by plants during photosynthesis • When plants and animals die carbon is transferred to rocks • Some forms fossil fuels which are released into the atmosphere when burnt The main gases in air can be separated out by fractional distillation. These gases are useful in industry
- 35. C1 7.6 CARBON DIOXIDE IN THE ATMOSPHERE The stages in the cycle are shown below: Carbon moves into and out of the atmosphere due to • Plants – photosynthesis & decay • Animals – respiration & decay • Oceans – store CO2 • Rocks – store CO2 and release it when burnt CO2 LEVELS Have increased in the atmosphere recently largely due to the amount of fossil fuels we now burn
