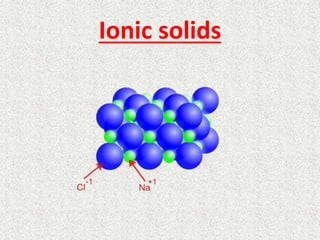
Ionic solids
- 1. Ionic solids
- 2. Introduction • Ionic Solids are solids composed of oppositely charged ions. They consist of positively charged cations and negatively charged anions. • In an ionic compound, the cations and anions are arranged in space to form an extended 3-D array that maximizes the number of attractive electrostatic interactions and minimizes the number of repulsive electrostatic interactions.
- 3. •The electrostatic energy of the interaction between two charged particles is proportional to the product of the charges on the particles and inversely proportional to the distance between them: electrostatic energy ∝ Q1Q2/ r
- 4. Properties of ionic solids • Ionic solids are generally high melting (more than 150 degrees C). Ionic solids are hard and brittle. Ionic solids melt to form liquids that are electrical conductors because the ions are free to move. • Ionic solids that are water soluble, dissolve to form solutions that are electrical conductors. • Ionic solids are brittle and hard because the electrostatic attractions in the solid again hold the ions in definite positions. • Ionic solids can be composed of simple ions as see in NaCl (sodium chloride) and like in ammonium nitrate NH4NO3 with NH4 + and NO3 - ions.
- 5. Radius ratio effect and coordination number • By using radius ratio rule, it is possible to predict the cation /anion coordination number in any compound. So radius ratio is a useful measure in establishing the structure of ionic solids. • radius ratio is the ratio of the ionic radius of the cation to the ionic radius of the anion in a cation- anion compound. • Relation between the radius, co-ordination number and the structural arrangement of the molecule. • radius ratio =
- 6. Structural analysis by radius ratio rule S.NO. RADIUS RATIO CO-ORDINATION NUMBER SHAPE EXAMPLE 1. 0.0 – 0.155 2 Linear HF- 2. 0.155–0.225 3 Triangular planar B 3. 0.225– 0.414 4 Tetrahedral ZnS 4. 0.414– 0.732 6 Octahedral NaCl 5. 0.732 – 1.0 8 Body-centered cubic CsCl
- 7. Example of radius ratio rule • Consider zinc sulphide in which Zinc ions thus prefer the tetrahedral holes in the close packed lattice of sulphide ions. • In the same way, we can predict that sodium ions will prefer octahedral holes in a close packed lattice of chloride ions . With larger cations, such as cesium, the ratio increases beyond the limit for a coordination number of 6 (0.414 - 0.732). Cesium ions now occupy cubic sites, i.e., coordination number of cations increases to 8 in a lattice of chloride ions .
- 8. Calculation of limiting radius ratio 1. Triangular planar cos30◦= CD/BD
- 10. 3.Tetrahedral • AB2 =BD2 +AD2 AB =r+ +r - and AD= r - In ∆ ACE AC2 =AE2 +EC2 BD=1/√2 r – put the value of BD in the main equation and we get radius ratio =0.225
- 11. • Limitation of the Radius Ratio Rule (i) Each ion is considered as a hard sphere for determining the optimum arrangement of ions in the crystal lattice. This is far form reality and serious errors can be made if anions get polarized and the bonding the intermediate (partially covalent) in character. (ii) Some compounds may crystallize in more than one modification with different coordination numbers. In such case, anion-anion repulsions and hence internuclear distance would be different. (iii) alkali metals halide • Effective radius of a cation is greatly influenced by the anions with the consequence that the radius ratio changes. For example, AgF and NaCl crystallize out in NaCl type of structure with coordination No. 6 and if we assume that the size of F- remains constant than Ag+ is bigger than Na+. On the other hand, for a given size for a chloride ion in AgCI and NaCI, the sizes of cations are reversed, that is, Na+ is bigger than Ag+. It is also true for their bromides. This is understandable because Ag+ is softer than N+ and introduces relatively more covalent character with Polarizable anions like CI- and Br-
