Chemical Equilibruim lecture 2 8 Jan.pdf
- 1. Chemical Equilibrium 11th Law of Mass ACTION
- 2. Welcome to the Class! Dear Learners, I’m MONICA BEDI Chemistry Educator (Decade+ Exp) Ph.D Chemistry TOPMOST Educator @ Unacademy EDUCATOR CODE MBLIVE GET 10% Off on Unacademy Subscription
- 3. Telegram Channel Unacademy Class 11 & 12 https://t.me/joinchat/QekesPpVTFswYmQ9
- 5. Days Class 11th Class 12th Monday 3 PM Tuesday 9 PM Wednesday* 8 PM JEE - Thursday 9 PM Friday 3 PM 9 PM Saturday 3 PM -
- 6. Unacademy Ask a Doubt Ask unlimited doubts Ask doubts at any time Get high-quality video solutions in English & Hindi Receive exact matches for questions Obtain instant and accurate solutions to lakhs of questions Get assistance with homework MBLIVE
- 12. Live polls & Leaderboard LIVE Doubt Clearing Sessions Unacademy Subscription LIVE Classes Interact with Educator
- 14. CBSE Class 11 Term 2 Batch Course
- 15. Starts: 7th Jan 2021 Day: M W F S Time: 7:15 pm Important for CLASS 11 TERM -2 Preparation Full Swing
- 18. EXCLUSIVE
- 19. Equilibrium is the state at which the concentrations of reactants and products do not change with time. This state can be recognized by the constancy of certain measurable properties such as pressure, density, colour, concentration, etc. What is Equilibrium
- 21. Dynamic equilibrium. Dynamic means moving and at a microscopic level, the system is in motion. There is no apparent change at equilibrium yet both forward and backward reactions continue to take place. Lets understand
- 22. Lets understand
- 23. Rate of forward reaction = Rate of backward reaction Lets understand
- 24. CONCEPT OF EQUILIBRIUM STATE Rate of forward reaction = Rate of backward reaction
- 25. 1. The observable properties of the system, such as pressure, colour, concentration, etc., become constant at equilibrium and remain unchanged thereafter. 2. The equilibrium can be approached from either direction. 3. The equilibrium can be attained only if the system is closed. 4. A catalyst does not alter the equilibrium point. CHARACTERISTICS OF CHEMICAL EQUILIBRIUM
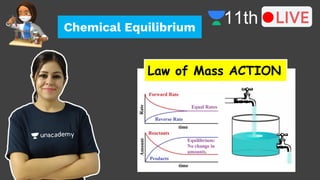
- 26. LAW OF MASS ACTION At constant temperature the rate of a chemical reaction is directly proportional to the product of the molar concentrations of reacting species with each concentration term raised to the power equal to the numerical co-efficient of that species in the chemical equation. LAW OF CHEMICAL EQUILIBRIUM AND EQUILIBRIUM CONSTANT
- 27. For any general reaction, aA + bB-------> Products The law of mass action may be written as Rate = k [A]a[B]b LAW OF MASS ACTION
- 28. LAW OF MASS ACTION Let us consider a simple reversible reaction,
- 29. LAW OF MASS ACTION
- 30. LAW OF MASS ACTION
- 31. LAW OF MASS ACTION
- 32. EQUILIBRIUM CONSTANT IN GASEOUS SYSTEMS
- 33. EQUILIBRIUM CONSTANT IN GASEOUS SYSTEMS
- 34. EQUILIBRIUM CONSTANT IN GASEOUS SYSTEMS
- 35. EQUILIBRIUM CONSTANT IN GASEOUS SYSTEMS
- 36. EQUILIBRIUM CONSTANT IN GASEOUS SYSTEMS
- 37. (i) N2(g) + 3H2 (g) ⇌ 2NH3 (g) Kp = Kc (RT)Dn (ii) H2 (g) + I2 (g) ⇌ 2HI (g) Dng = 2 – (1 + 1) = 0 (iii) 2 NOCI (g) ⇌ 2NO (g) + Cl2 (g) Dng = 2 – (1 + 1) = 0 g Lets Practice
- 38. By applying law of mass action to a reversible reaction, at equilibrium, it is possible to derive a simple mathematical expression known as law of chemical equilibrium. Let us consider a simple reversible reaction, A + B C + D Rate of forward reaction [A][B] = kf [A][B] where kf is the rate constant for the forward reaction. Similarly, Rate of backward reaction [C][D] = kb[C][D] where kb is the rate constant for the backward. LAW OF MASS ACTION: Application
- 39. Rate of forward reaction = Rate of backward reaction. kf[A][B] = kb[C][D] or Since kf and kb are constants, therefore, the ratio kf/kb is also constant and is represented by Kc. f b k C D k A B f c b k C D K k A B LAW OF MASS ACTION: Application
- 40. The constant, Kc is called equilibrium constant. For a general reaction of the type : aA + bB cC + dD The equilibrium constant, Kc, may be defined as the ratio of product of the equilibrium concentrations of the products to that of the reactants with each concentration term raised to the power equal to the stoichio-metric co-efficient of the substance in the balanced chemical equation. c d c a b C D K A B Equilibrium Constant
- 41. Write the equilibrium constant expressions for the following reactions: (a) BaCO3(s) BaO(s) + CO2(g) (b) AgBr(s) Ag+ (aq) + Br– (aq) (c) CH3COCH3(l) CH3COCH3(g) (d) CH4(g) + 2O2(g) CO2(g) + 2H2O(g) Lets Practice
- 42. Law of chemical equilibrium may be stated as : At a given temperature, the ratio of product of equilibrium concentrations of the products to that of the reactants with each concentration term raised to the power equal to the respective stoichiometric co-efficients in the balanced chemical has a constant value. The concentration ratio = c d a b C D A B Equilibrium Constant
- 43. If an equilibrium involves gaseous species, then the concentrations in the concentration quotient may be replaced by partial pressures because at a given temperature the partial pressure of a gaseous component is proportional to its concentration. If the above mentioned reaction has all the gaseous species, then c d C D p p a b A B p p Q K p p EQUILIBRIUM CONSTANT IN GASEOUS SYSTEMS
- 44. For an ideal gas, PV = nRT p = n/V RT = CRT pa = CART pB = CBRT pC = CCRT pD = CDRT Kp = Kc(RT)Dn Dn = (sum of the exponents in the numerator of Qc) – (sum of the exponents in the denominator of Qc) c d C D P a b A B C RT C RT K C RT C RT . c d c d a b C D a b A B C C RT C C RELATIONSHIP BETWEEN Kp AND Kc
- 45. 1. At equilibrium, the concentrations of N2, O2 and NO in a sealed vessel at 800 K are : N2 = 3.0 x 10–3 M, O2 = 4.2 x 10–3 M and NO = 2.8 x 10–3 M Calculate the equilibrium constant Kc for the reaction: N2(g) + O2(g) ⇌ 2NO(g) Lets Practice
- 46. 2. What is Kc for the following equilibrium, when the equilibrium concentration of each substance is [SO2] = 0.60 M, (O2) = 0.82 M and (SO3] = 1.90 M 2SO2(g) + O2(g) ⇌ 2SO3(g) Lets Practice
- 47. 3. PCl5, PCl3 and Cl2 are at equilibrium at 500 K and having concentration 1.59 M PCl3, 1.59 M Cl2 and 1.41 M PCl5. Calculate Kc for the reaction : PCl5 ⇌ PCl3 + Cl2 Lets Practice
- 49. Days Class 11th Class 12th Monday 3 PM Tuesday 9 PM Wednesday* 8 PM JEE - Thursday 9 PM Friday 3 PM 9 PM Saturday 3 PM -
- 50. Unacademy Ask a Doubt Ask unlimited doubts Ask doubts at any time Get high-quality video solutions in English & Hindi Receive exact matches for questions Obtain instant and accurate solutions to lakhs of questions Get assistance with homework MBLIVE
- 56. Live polls & Leaderboard LIVE Doubt Clearing Sessions Unacademy Subscription LIVE Classes Interact with Educator
- 58. CBSE Class 11 Term 2 Batch Course
- 59. Starts: 7th Jan 2021 Day: M W F S Time: 7:15 pm Important for CLASS 11 TERM -2 Preparation Full Swing
- 62. EXCLUSIVE
- 63. Step 2 Step 1 Step 2 Step 3 Step 4 Step 5
- 64. EXCLUSIVE