Membrane transport
- 1. Membrane Transport Represented by_ Saurav K. Rawat (M.sc. Chem.) (Physical Special)
- 2. Department of Chemistry St. John’s College , Agra
- 3. Membrane transport: The set of transport proteins in the plasma membrane, or in the membrane of an intracellular organelle, determines exactly what solutes can pass into and out of that cell or organelle. Each type of membrane therefore has its own characteristic set of transport proteins. Each type of transport protein transports a particular type of molecules – selective set of solute are transported in or out
- 4. 3 Types of transport
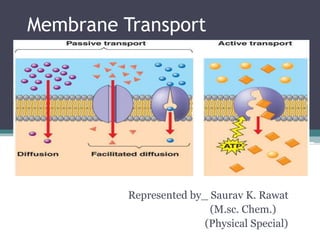
- 5. Passive Transport Simple diffusion ▫ Small non-polar molecules No ions ▫ Examples: Fatty acids Steroids CO2 O2 H2O (?)—osmosis ▫ Only move DOWN concentration gradient
- 7. Osmosis
- 9. Mediated Transport • Passive mediated transport ▫ Facilitated diffusion Carrier proteins Channel proteins ▫ DOWN a conc. gradient • Active transport ▫ Primary active transport—uses ATP ▫ Secondary active transport—uses a different energy source ▫ Pumps things UP a conc. gradient
- 10. How to tell mediated transport vs. simple diffusion • Saturation kinetics • Competition kinetics • Specificity
- 11. Some more terms
- 12. GluT1 (RBCs)—a carrier protein
- 13. Channel proteins • Ion channels ▫ Ions ▫ Selective ▫ Generally gated • Porins ▫ Larger ▫ Less specific • Aquaporins ▫ water
- 14. Direct and Indirect AT
- 16. The Na/K ATPase
- 18. Consequences of Na/K ATPase • Two ion gradients ▫ Used as energy source ▫ Electrical signaling • Charge difference across membrane ▫ Membrane potential difference ▫ Negative on inside -60 to –90 mV in animal cells ~ -150 mV in bacteria -200 to –300 mV in plants ▫ Not just due to these ions Phosphatidylserine on inside of PM Other ions
- 19. Indirect (secondary) AT • Na/glucose symporter ▫ Intestinal absorptive cells
- 22. Thermodynamic treatment Mass transport across the membrane must be discussed fundamentally on the basis of the thermodynamics because the thermodynamics describes the rule of energy changes inevitably generating in the mass transport. The classical thermodynamics have introduced a lot of information on the basis of the first and the second law. However, we have to notice that the classical thermodynamics discusses only reversible phenomena and it does not treats the transport rate, in other words, it does not includes the parameter of “time”. Many phenomena (including membrane phenomena) generating in the natural world are far away from reversible states, so the reversible thermodynamics was ineffective to discuss their mechanism. On other hand, the irreversible thermodynamics (Non-equilibrium thermo-dynamics) came to succeed in discussing the mass transport by introducing the concept of “time” in its system. The irreversible thermodynamics is not general theory applicable to analyze every reaction in the natural world. Further it should be noticed that the applicable limit of the irreversible thermodynamics must be determined through experiments.
- 23. Biochemistry: 09/25/08 Lipid2/Membranes p. 23 of 41 Membrane Transport • What goes through and what doesn’t? • Nonpolar gases (CO2, O2) diffuse • Hydrophobic molecules and small uncharged molecules mostly pass freely • Charged molecules blocked
- 24. Biochemistry: 09/25/08 Lipid2/Membranes p. 24 of 41 Transmembrane Traffic: Types of Transport (Table 9.3) Type ProteinSaturable Movement Energy Carrierw/substr. Rel.to conc. Input? Diffusion No No Down No Channels Yes No Down No & pores Passive Yes Yes Down No transport Active Yes Yes Up Yes
- 25. Biochemistry: 09/25/08 Lipid2/Membranes p. 25 of 41 Cartoons of transport types • From accessexcellence.org
- 26. Biochemistry: 09/25/08 Lipid2/Membranes p. 26 of 41 Thermodynamics of passive and active transport • If you think of the transport as a chemical reaction Ain Aout or Aout Ain • It makes sense that the free energy equation would look like this: • Gtransport = RTln([Ain]/[Aout]) • More complex with charges; see eqns. 9.4 through 9.6.
- 27. Biochemistry: 09/25/08 Lipid2/Membranes p. 27 of 41 Example • Suppose [Aout] = 145 mM, [Ain] = 10 mM, T = body temp = 310K • Gtransport = RT ln[Ain]/[Aout] = 8.325 J mol-1K-1 * 310 K * ln(10/145) = -6.9 kJ mol-1 • So the energies involved are moderate compared to ATP hydrolysis
- 28. Biochemistry: 09/25/08 Lipid2/Membranes p. 28 of 41 Charged species • Charged species give rise to a factor that looks at charge difference as well as chemical potential (~concentration) difference • Most cells export cations so the inside of the cell is usually negatively charged relative to the outside
- 29. Biochemistry: 09/25/08 Lipid2/Membranes p. 29 of 41 Quantitative treatment of charge differences • Membrane potential (in volts J/coul): Y = Yin - Yout • Gibbs free energy associated with change in electrical potential is Ge = zFY where z is the charge being transported and F is Faraday’s constant, 96485 JV-1mol-1 • Faraday’s constant is a fancy name for 1.
- 30. Biochemistry: 09/25/08 Lipid2/Membranes p. 30 of 41 Faraday’s constant • Relating energy per mole to energy per coulomb: • Energy per mole of charges, e.g. 1 J mol-1, is 1 J / (6.022*1023 charges) • Energy per coulomb, e.g, 1 V = 1 J coul-1, is 1 J / (6.241*1018 charges) • 1 V / (J mol-1) = (1/(6.241*1018)) / (1/(6.022*1023) = 96485 • So F = 96485 J V-1mol-1
- 31. Biochemistry: 09/25/08 Lipid2/Membranes p. 31 of 41 Total free energy change • Typically we have both a chemical potential difference and an electrical potential difference so • Gtransport = RTln([Ain]/[Aout]) + zFY • Sometimes these two effects are opposite in sign, but not always
- 32. Biochemistry: 09/25/08 Lipid2/Membranes p. 32 of 41 Pores and channels • Transmembrane proteins with central passage for small molecules, possibly charged, to pass through ▫ Bacterial: pore. Usually only weakly selective ▫ Eukaryote: channel. Highly selective. • Usually the Gtransport is negative so they don’t require external energy sources • Gated channels: ▫ Passage can be switched on ▫ Highly selective, e.g. v(K+) >> v(Na+) Rod MacKinnon
- 33. Biochemistry: 09/25/08 Lipid2/Membranes p. 33 of 41 Protein-facilitated passive transport • All involve negative Gtransport ▫ Uniport: 1 solute across ▫ Symport: 2 solutes, same direction ▫ Antiport: 2 solutes, opposite directions • Proteins that facilitate this are like enzymes in that they speed up reactions that would take place slowly anyhow • These proteins can be inhibited, reversibly or irreversibly Diagram courtesy Saint-Boniface U.
- 34. Energetics of Transport Ain Aout GA = RT ln ([A]in/[A]out) if [A]out>[A]in, then G<0 for inward movement
- 35. Thermodynamics of Transport, charged GA = RT ln ([A]in/[A]out) + ZA F Z= charge on A F = Faraday's constant, the charge in a mole of electrons Y = membrane potential, difference in charge between in and out, generally negative
- 36. Two major classes of membrane transmembrane proteins Carrier proteins bind a solute on one side and deliver it to the other side through a change in shape. Cells can also transport macromolecules across the membrane Channel proteins form tiny hydrophilic pores in the membrane and the specific molecules pass through by diffusion from high to low concentration. Most are ion channels
- 37. The ion concentrations inside a cell are very different from those outside Because ions are electrically charged, their movements can create powerful electric forces across the membrane. Important in nerve cells, muscle cells, and in the mitochondria, for ATP synthesis using the electron transport chain.
- 38. Ion transport across cell membranes is of central importance in biology. Cells maintain an internal ion composition very different from that in the fluid around them and these differences are crucial for the cell’s survival and function. Animal cells pump Na+ out. If the pumping fails, water flows in by osmosis and causes the cell to swell and burst. The positive and negative charges must be balanced by an almost exactly equal quantity both inside and outside of the cell Differential ion concentrations crucial. nucleic acids, proteins, etc.
- 39. Carrier proteins are required for the transport of almost all small organic molecules across the cell membranes. Each carrier is highly selective, often transporting just one type of molecule. ADP Each cell, each organelle has particular transport proteins.
- 40. The membrane transport proteins studied have polypeptide chains that traverse the lipid bilayer multiple times, forming a continuous protein-lined pathway allowing selected small hydrophilic molecules to cross without coming into contact with the hydrophobic lipid bilayer. A basic difference between carrier proteins and channel proteins is the way they discriminate between solutes. Channel proteins mainly go on size and electric charge. Carrier proteins actually bind their molecules it transfers, and then changes conformation
- 41. Passive transport – no energy needed, with the concentration gradient Active transport - energy needed, against the concentration gradient Passive transport with a carrier protein = facilitated transport
- 42. Passive transport of glucose: carrier exists in at least to states (shapes). Example: liver cell after a large meal – lots of glucose outside in the extracellular fluid. Opposite in liver when blood glucose becomes low. Liver cells breakdown glycogen. Glucose levels are higher inside the cell now. Passive transport moves glucose outside the cell.
- 43. Electrically charged molecules diffuse according to their concentration gradient and according to their charge – electrochemical gradient. Membranes typically have a voltage difference across them. Negative inside. steep gradient Na+ is at a higher concentration outside of the cell so it tends to enter the cell if given the chance. inside outside K+ is present at a higher concentration inside the cell – therefore there is little movement
- 44. Active transport moves solutes against their electrochemical gradients. against with
- 45. Animal cells use the Energy of ATP hydrolysis to pump Na+ out of the cell to maintain the electrochemical gradient. This gradient is then used to pump other molecules into or out of the cell against their elecrochemical gradient. operates ceaselessly Na+-K+ ATPase or Na+-K+ pump other ATP driven pumps create electrochemical gradients of H+ ions. Next chapter
- 46. Na+-K+ ATPase or Na+-K+ pump
- 47. Animal cells use the Na+ gradient to take up nutrients actively.
- 49. The Na+-K+ pump helps maintain the osmotic balance of animal cells.
- 51. • Cytosolic Ca+2 concentrations are kept low by Ca+2 pumps. • Influx of Ca+2 is tightly regulated, since Ca+2 binds molecules (enzymes) and alters their activities (activation or inhibition). • Influx of Ca+2 through Ca+2 channels is often used as a signal to trigger other intracellular events (muscle contraction). • The cell maintains a low concentration, so that signaling via increases is kept sensitive.
- 52. Ion channels are ion selective and gated. Tthey show ion selectivity depending on the diameter and shape of the ion channel and on the distribution of charged amino acids in its lining.. Most ion channels are gated: they can switch between an open and a closed state by a change in conformation, which is regulated by conditions inside and outside the cell.
- 53. Ion channels are important in signaling in neurons
- 54. If the plasma membrane of animal cells was made permeable to Na+ and K+, the Na+-K+ pump would: A. be completely inhibited. B. begin to pump Na+ in both directions. C. begin synthesizing ATP instead of hydrolyzing it. D. continue to pump ions and to hydrolyze ATP but the energy of hydrolysis would be wasted, as it would generate heat rather than ion gradients. E. continue to pump ions but would not hydrolyze ATP.
- 55. Ca2+ pumps in the plasma membrane and endoplasmic reticulum are important for: A. maintaining osmotic balance. B. preventing Ca2+ from altering the behavior of molecules in the cytosol. C. providing enzymes in the endoplasmic reticulum with Ca2+ ions that are necessary for their catalytic activity. D. maintaining a negative membrane potential. E. helping cells import K+.
- 56. Rawat’s Creation-rwtdgreat@ gmail.com rwtdgreat@yahoo.co.uk RawatDAgreatt/LinkedIn www.slideshare.net/ RawatDAgreatt Google+/blogger/Facebook/ Twitter-@RawatDAgreatt +919808050301 +919958249693
