batteries class.pptx
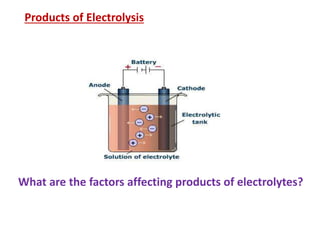
- 1. Products of Electrolysis What are the factors affecting products of electrolytes?
- 2. Products of Electrolysis- Factors a) Nature of the electrode b) Reduction potential of ions Types of electrodes Active Takes part in reaction (Cu, Ni, Ag) Inert do not take part in reaction(Pt, C)
- 3. Predict the products of electrolysis at cathode & anode. a) Molten sodium chloride is electrolyzed using Pt electrode. Cathode: Sodium metal Anode: Chlorine gas
- 4. Predict the products b) Molten Copper chloride is electrolyzed using Cu electrode. Cathode: Copper metal Anode: Cu2+
- 5. Products of Electrolysis- Factors b) Reduction potential of ions A reaction occurs at cathode higher 0E anode lower 0E
- 6. Predict the products of electrolysis of an aqueous solution of sodium chloride using Pt electrode. Cathode reaction Hydrogen gas is liberated
- 7. Products of Electrolysis – continued ….. Predict the products at anode when NaCl (aq) is electrolyzed? Anode Liberation of chlorine gas takes place Over potential The minimum excess potential over the standard reduction potential require to discharge an ion.
- 9. One or more galvanic cells connected in series and used as a source of electricity Compact Light weight Constant voltage
- 11. Dry cell(Leclanche cell) Anode: Zinc container Cathode: Graphite + MnO2 Electrolyte: NH4Cl + ZnCl2 paste Cell potential = 1.5 V Primary battery 1
- 12. Mercury Cell Anode: Zn(Hg) Cathode: HgO + C Electrolyte: KOH + ZnO (Paste) Primary battery 2
- 13. Mercury Cell Cell potential = 1.35 V (constant voltage)
- 14. Anode: Pb Cathode: PbO2 + Pb Electrolyte: H2SO4(38%) Lead storage Battery Secondary Battery 1
- 15. Cell potential = 2 V (per cell) Lead storage Battery Battery in use On Charging the Battery Reverse above reaction
- 16. Anode: Cd Cathode: Ni(OH)3 Electrolyte: KOH Cell potential = 1.4 V Nickel Cadmium Battery Secondary Battery 2 Overall reaction
- 17. HOME WORKS a) Write the uses of primary & secondary cells b) Write the overall reaction taking place in those cells
- 18. Fuel cells Galvanic cells which convert energy of combustion of fuel directly into electrical energy are called fuel cells e.g. Hydrogen – Oxygen fuel cell Methanol – Oxygen fuel cell
- 19. Hydrogen – Oxygen fuel cell NaOH
- 20. Rusting
- 21. Rusting A process in which iron reacts with water and oxygen from its surrounding to form hydrated ferric oxide(rust).Fe2O3.xH2O Rusting – a redox reaction
- 22. Do other metals react with water or air in its surrounding? It is the gradual destruction of metals by electrochemical reaction with their environment Corrosion
- 23. Prevention of rusting 1) Painting 2) Galvanizing(Zn Coating) 3) Cathodic protection
- 25. REVISION- ELECTROCHEMISTRY Pattern Max. Marks:8 Part A 1 Mark x 1 Question Part B 2 Marks x 1 Question Part D 5 Marks x 1 question
- 26. REVISION- ELECTROCHEMISTRY Important topics 1. Daniel cell 2. SHE 3. Nernst equation problems 4. Conductivity and molar conductivity 5. Kohlrausch’s law 6. Faraday’s laws 7. Batteries ( Dry cell, Pb Acid Battery) 8. Fuel cell 9. Rusting of iron
- 27. 1) Explain the construction and working of SHE 2) Define Molar conductivity 3) State Kohlrausch’s law 4) State Faraday’s first law and second law 5) Explain the construction and working of H2-O2 Fuel cell. 6) Explain cathode and anode reactions in a Dry cell 7) Explain the over all reaction in a Lead storage battery when it is in use. Learn – Answer - Write
- 28. The Gibb’s energy for the decomposition of aluminium oxide at 500 oC is –966 kJmol-1. What is the minimum potential difference needed for electrolytic decomposition of Al2O3. ⅔ Al2O3 4/3 Al + 3O2 Number of electrons involved = n ⅔ Al3+ 4/3 Al0 hence (2/3)* 3 = 2 e and (4/3) * 0 = 0e Number of electrons per aluminium atom is = 2 Hence for two aluminium atoms = 4 electrons n = 4 ∆G0 = -nFE0 Hence E0 = - ∆G0 /nF On substituting the values E0 cell = - (-966kJ/4 * 96500) Minimum potential difference = 2.5 V
- 29. Calculate the emf of the cell Fe/Fe2+(0.6M)//Sn2+(0.2M)/Sn E0 Fe = -0.44V and E0 Sn = + 0.14 V Answer: n = 2 E0cell = 0.58 V Emf of cell = 0.566 V
- 30. 0.2964 g of copper was deposited on passage of a current of 0.5 A for 30 minutes through a solution of copper sulphate. Calculate the atomic mass of copper. Answer: Atomic mass of copper = 63.56
Notas do Editor
- 4OH- 2H2O + O2
- Put pictures of different batteries
- Show cells connected and show it is small and compact compare with a big daniel cell
- Electrochemical reactions with non-reversible materials in the electrodes are utilized, therefore cannot regenerate electricity
- Put video and picture ammonia trapped by Zn2+ to form complex [Zn(NH3)4]2+
- Put video and picture
- Put video and picture
- Put video and picture
- Put video and picture
- Cd + OH- CdO + H2O 2Ni(OH)3 + 2e 2Ni(OH)2 + 2OH-
- By product of methanol oxygen fuel cell will be CO2 and H2O
- Explain and use the video