Changing the Paradigm of CMV Management: New Science and More Choices for Challenging Cases in the HCT Setting
•
0 gostou•43 visualizações
Roy F. Chemaly, MD, MPH, FIDSA, FACP, and Genovefa Papanicolaou, MD, FIDSA, prepared useful practice aids pertaining to cytomegalovirus for this CME activity titled "Changing the Paradigm of CMV Management: New Science and More Choices for Challenging Cases in the HCT Setting." For the full presentation, monograph, complete CME information, and to apply for credit, please visit us at http://bit.ly/38UNLwZ. CME credit will be available until March 19, 2021.
Denunciar
Compartilhar
Denunciar
Compartilhar
Baixar para ler offline
Recomendados
Chair Jamie Carroll, APRN, CNP, MSN, discusses breast cancer in this NCPD/ILNA/AAPA activity titled “Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy in TNBC and HR+, HER2- Breast Cancer: Best Practices for Adverse Event Management and Patient Education.” For the full presentation, downloadable Practice Aids, and complete NCPD/ILNA/AAPA information, and to apply for credit, please visit us at https://bit.ly/3SdnvWt. NCPD/ILNA/AAPA credit will be available until May 8, 2025.Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy...

Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy...PVI, PeerView Institute for Medical Education
Chair Jonathan A. Bernstein, MD, discusses chronic spontaneous urticaria in this CME activity titled “BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Treatment.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/3P0cnvi. CME credit will be available until May 6, 2025.BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Tr...

BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Tr...PVI, PeerView Institute for Medical Education
Co-Chairs Milind Desai, MD, MBA, FACC, FAHA, FESC, and Andrew Willeford, PharmD, PhD, BCCP, prepared useful Practice Aids pertaining to hypertrophic cardiomyopathy for this CME/MOC/CPE/AAPA/IPCE activity titled “Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertrophic Cardiomyopathy.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/4bb7eKc. CME/MOC/CPE/AAPA/IPCE credit will be available until May 16, 2025.Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...

Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...PVI, PeerView Institute for Medical Education
Co-Chairs Milind Desai, MD, MBA, FACC, FAHA, FESC, and Andrew Willeford, PharmD, PhD, BCCP, discuss hypertrophic cardiomyopathy in this CME/MOC/CPE/AAPA/IPCE activity titled “Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertrophic Cardiomyopathy.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/4bb7eKc. CME/MOC/CPE/AAPA/IPCE credit will be available until May 16, 2025.Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...

Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...PVI, PeerView Institute for Medical Education
Chair A. Michael Lincoff, MD, discusses obesity in this CME activity titled “Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Weight Loss Pharmacotherapy.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/3KAO98K. CME credit will be available until April 25, 2025.Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Wei...

Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Wei...PVI, PeerView Institute for Medical Education
Co-Chairs and Planners Saakshi Khattri, MBBS, MD, FAAD, FACR, Marla Dubinsky, MD, Emma Guttman-Yassky, MD, PhD, and Alexis Ogdie, MD, MSCE, discuss immune-mediated inflammatory diseases in this CME/AAPA activity titled “Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Diseases: Addressing Shared Pathophysiology With JAK Inhibitors.” For the full presentation, downloadable Practice Aids, and complete CME/AAPA information, and to apply for credit, please visit us at https://bit.ly/3JhsIZ7. CME/AAPA credit will be available until April 24, 2025.Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Di...

Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Di...PVI, PeerView Institute for Medical Education
Co-Chairs Alicia K. Morgans, MD, MPH, and Neal D. Shore, MD, FACS, discuss prostate cancer in this CME/MOC/NCPD/CPE/AAPA/IPCE activity titled “Treatment Advances and Individualized Therapeutic Strategies in Prostate Cancer: Expert Insights on Key Evidence, Practical Tips for Personalized Therapy, and Clinical Integration Approaches.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3SQrJ6G. CME/MOC/NCPD/CPE/AAPA/IPCE credit will be available until April 24, 2025.Treatment Advances and Individualized Therapeutic Strategies in Prostate Canc...

Treatment Advances and Individualized Therapeutic Strategies in Prostate Canc...PVI, PeerView Institute for Medical Education
Co-Chairs Prof. Nicolas Girard, MD, PhD, and Aaron Lisberg, MD, discuss NSCLC in this CME/MOC/NCPD/AAPA/IPCE activity titled “Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Cancer: Unveiling Potential, Shaping Tomorrow.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3RmX3dU. CME/MOC/NCPD/AAPA/IPCE credit will be available until April 24, 2025.Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Canc...

Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Canc...PVI, PeerView Institute for Medical Education
Recomendados
Chair Jamie Carroll, APRN, CNP, MSN, discusses breast cancer in this NCPD/ILNA/AAPA activity titled “Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy in TNBC and HR+, HER2- Breast Cancer: Best Practices for Adverse Event Management and Patient Education.” For the full presentation, downloadable Practice Aids, and complete NCPD/ILNA/AAPA information, and to apply for credit, please visit us at https://bit.ly/3SdnvWt. NCPD/ILNA/AAPA credit will be available until May 8, 2025.Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy...

Nurses at the Forefront of Maximizing the Potential of TROP2-Targeted Therapy...PVI, PeerView Institute for Medical Education
Chair Jonathan A. Bernstein, MD, discusses chronic spontaneous urticaria in this CME activity titled “BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Treatment.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/3P0cnvi. CME credit will be available until May 6, 2025.BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Tr...

BTK Inhibition Transforming the Landscape of Chronic Spontaneous Urticaria Tr...PVI, PeerView Institute for Medical Education
Co-Chairs Milind Desai, MD, MBA, FACC, FAHA, FESC, and Andrew Willeford, PharmD, PhD, BCCP, prepared useful Practice Aids pertaining to hypertrophic cardiomyopathy for this CME/MOC/CPE/AAPA/IPCE activity titled “Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertrophic Cardiomyopathy.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/4bb7eKc. CME/MOC/CPE/AAPA/IPCE credit will be available until May 16, 2025.Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...

Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...PVI, PeerView Institute for Medical Education
Co-Chairs Milind Desai, MD, MBA, FACC, FAHA, FESC, and Andrew Willeford, PharmD, PhD, BCCP, discuss hypertrophic cardiomyopathy in this CME/MOC/CPE/AAPA/IPCE activity titled “Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertrophic Cardiomyopathy.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/4bb7eKc. CME/MOC/CPE/AAPA/IPCE credit will be available until May 16, 2025.Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...

Adapting Managed Care Strategies in the Era of Precision Medicine for Hypertr...PVI, PeerView Institute for Medical Education
Chair A. Michael Lincoff, MD, discusses obesity in this CME activity titled “Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Weight Loss Pharmacotherapy.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/3KAO98K. CME credit will be available until April 25, 2025.Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Wei...

Exploring the Evidence: Improving Cardiovascular Outcomes and the Role of Wei...PVI, PeerView Institute for Medical Education
Co-Chairs and Planners Saakshi Khattri, MBBS, MD, FAAD, FACR, Marla Dubinsky, MD, Emma Guttman-Yassky, MD, PhD, and Alexis Ogdie, MD, MSCE, discuss immune-mediated inflammatory diseases in this CME/AAPA activity titled “Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Diseases: Addressing Shared Pathophysiology With JAK Inhibitors.” For the full presentation, downloadable Practice Aids, and complete CME/AAPA information, and to apply for credit, please visit us at https://bit.ly/3JhsIZ7. CME/AAPA credit will be available until April 24, 2025.Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Di...

Interdisciplinary Approaches to Management of Immune-Mediated Inflammatory Di...PVI, PeerView Institute for Medical Education
Co-Chairs Alicia K. Morgans, MD, MPH, and Neal D. Shore, MD, FACS, discuss prostate cancer in this CME/MOC/NCPD/CPE/AAPA/IPCE activity titled “Treatment Advances and Individualized Therapeutic Strategies in Prostate Cancer: Expert Insights on Key Evidence, Practical Tips for Personalized Therapy, and Clinical Integration Approaches.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3SQrJ6G. CME/MOC/NCPD/CPE/AAPA/IPCE credit will be available until April 24, 2025.Treatment Advances and Individualized Therapeutic Strategies in Prostate Canc...

Treatment Advances and Individualized Therapeutic Strategies in Prostate Canc...PVI, PeerView Institute for Medical Education
Co-Chairs Prof. Nicolas Girard, MD, PhD, and Aaron Lisberg, MD, discuss NSCLC in this CME/MOC/NCPD/AAPA/IPCE activity titled “Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Cancer: Unveiling Potential, Shaping Tomorrow.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3RmX3dU. CME/MOC/NCPD/AAPA/IPCE credit will be available until April 24, 2025.Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Canc...

Charting a New Path to Better Outcomes With TROP2-Targeting ADCs in Lung Canc...PVI, PeerView Institute for Medical Education
Co-Chairs, Joseph K. Han, MD, and Seth J. Isaacs, MD, prepared useful Practice Aids pertaining to chronic rhinosinusitis with nasal polyps for this CME/MOC/CC/AAPA/IPCE activity titled “Biologics in CRSwNP: Putting a Paradigm Shift Into Practice.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3Tq6n1G. CME/MOC/CC/AAPA/IPCE credit will be available until May 6, 2025.Biologics in CRSwNP: Putting a Paradigm Shift Into Practice

Biologics in CRSwNP: Putting a Paradigm Shift Into PracticePVI, PeerView Institute for Medical Education
Co-Chairs, Joseph K. Han, MD, and Seth J. Isaacs, MD, discuss chronic rhinosinusitis with nasal polyps in this CME/MOC/CC/AAPA/IPCE activity titled “Biologics in CRSwNP: Putting a Paradigm Shift Into Practice.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3Tq6n1G. CME/MOC/CC/AAPA/IPCE credit will be available until May 6, 2025.Biologics in CRSwNP: Putting a Paradigm Shift Into Practice

Biologics in CRSwNP: Putting a Paradigm Shift Into PracticePVI, PeerView Institute for Medical Education
Co-Chairs, R. Donald Harvey, PharmD, BCOP, FCCP, FHOPA, FASCO, Zahra Mahmoudjafari, PharmD, MBA, BCOP, FHOPA, and James Davis, PharmD, BCOP, discuss multiple myeloma in this CME/CPE/IPCE activity titled “Prescriptions for Successful Myeloma Care: Pharmacy Strategies for Delivering Effective Therapy With Antibody Platforms.” For the full presentation, downloadable Practice Aids, and complete CME/CPE/IPCE information, and to apply for credit, please visit us at https://bit.ly/4aa0iMX. CME/CPE/IPCE credit will be available until May 2, 2025.Prescriptions for Successful Myeloma Care: Pharmacy Strategies for Delivering...

Prescriptions for Successful Myeloma Care: Pharmacy Strategies for Delivering...PVI, PeerView Institute for Medical Education
Co-Chairs, Carlos G. Romo, MD, and Aimee Sato, MD, discuss Neurofibromatosis in this CME/MOC activity titled “Precision & Progress Against NF1: Solutions for Better Outcomes With MEKi & Multimodal Care for NF1 pNF and Other Tumors.” For the full presentation, downloadable Practice Aids, and complete CME/MOC information, and to apply for credit, please visit us at https://bit.ly/3SZRz8p. CME/MOC credit will be available until May 2, 2025.Precision & Progress Against NF1: Solutions for Better Outcomes With MEKi & M...

Precision & Progress Against NF1: Solutions for Better Outcomes With MEKi & M...PVI, PeerView Institute for Medical Education
Chair and Presenters Kathleen N. Moore, MD, MS, Floor J. Backes, MD, and Bhavana Pothuri, MD, MS, prepared useful Practice Aids pertaining to endometrial cancer for this CME/MOC/NCPD/AAPA/IPCE activity titled “Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Potential of Immunotherapy, ADCs, PARP Inhibitors, and Other Emerging Treatment Strategies.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3SjJyuH. CME/MOC/NCPD/AAPA/IPCE credit will be available until April 17, 2025.Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...

Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...PVI, PeerView Institute for Medical Education
Chair and Presenters Kathleen N. Moore, MD, MS, Floor J. Backes, MD, and Bhavana Pothuri, MD, MS, discuss endometrial cancer in this CME/MOC/NCPD/AAPA/IPCE activity titled “Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Potential of Immunotherapy, ADCs, PARP Inhibitors, and Other Emerging Treatment Strategies.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3SjJyuH. CME/MOC/NCPD/AAPA/IPCE credit will be available until April 17, 2025.Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...

Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...PVI, PeerView Institute for Medical Education
Chair and Presenters Bradley J. Monk, MD, FACS, FACOG, Kathleen N. Moore, MD, MS, and Ana Oaknin, MD, PhD, discuss gynecologic cancers in this CME/MOC/NCPD/AAPA/IPCE activity titled “Advancing ADCs in Gynecologic Cancers: Expert Insights on Recent Evidence, Implementation Strategies, and Patient Care.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/4a56tly. CME/MOC/NCPD/AAPA/IPCE credit will be available until April 16, 2025.Advancing ADCs in Gynecologic Cancers: Expert Insights on Recent Evidence, Im...

Advancing ADCs in Gynecologic Cancers: Expert Insights on Recent Evidence, Im...PVI, PeerView Institute for Medical Education
Chair Lecia V. Sequist, MD, MPH, and Patrick Nana-Sinkam, MD, FCCP, prepared useful Practice Aids pertaining to lung cancer for this CME/MOC/AAPA/IPCE activity titled “Screening and Early Intervention as the Keys to Success in Lung Cancer: A Practical Approach to Implementing Lung Cancer Screening for High-Risk Individuals.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/46VvwoP. CME/MOC/AAPA/IPCE credit will be available until April 16, 2025.Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...

Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...PVI, PeerView Institute for Medical Education
Chair Lecia V. Sequist, MD, MPH, and Patrick Nana-Sinkam, MD, FCCP, discuss lung cancer screening in this CME/MOC/AAPA/IPCE activity titled “Screening and Early Intervention as the Keys to Success in Lung Cancer: A Practical Approach to Implementing Lung Cancer Screening for High-Risk Individuals.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/46VvwoP. CME/MOC/AAPA/IPCE credit will be available until April 16, 2025.Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...

Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...PVI, PeerView Institute for Medical Education
Chair and Presenter, Olalekan Oluwole, MBBS, MPH, Veronika Bachanova, MD, PhD, and David L. Porter, MD, prepared useful Practice Aids pertaining to CAR-T therapy for this CME/NCPD activity titled “Democratizing the CAR-T Experience: The Principles and Practice of Outpatient Cellular Therapy.” For the full presentation, downloadable Practice Aids, and complete CME/NCPD information, and to apply for credit, please visit us at https://bit.ly/3TfIABM. CME/NCPD credit will be available until April 15, 2025.Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...

Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...PVI, PeerView Institute for Medical Education
Chair and Presenter, Olalekan Oluwole, MBBS, MPH, Veronika Bachanova, MD, PhD, and David L. Porter, MD, discuss CAR-T therapy in this CME/NCPD activity titled “Democratizing the CAR-T Experience: The Principles and Practice of Outpatient Cellular Therapy.” For the full presentation, downloadable Practice Aids, and complete CME/NCPD information, and to apply for credit, please visit us at https://bit.ly/3TfIABM. CME/NCPD credit will be available until April 15, 2025.Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...

Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...PVI, PeerView Institute for Medical Education
Co-Chairs Lipika Goyal, MD, MPhil, and Riad Salem, MD, MBA, discuss HCC in this CME activity titled “The Convergence of Interventional Radiologists and Oncologists in HCC: Shared Decision-Making and Care Coordination at the Center of Personalized Care Across the Disease Continuum.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/48BAasz. CME credit will be available until April 26, 2025.The Convergence of Interventional Radiologists and Oncologists in HCC: Shared...

The Convergence of Interventional Radiologists and Oncologists in HCC: Shared...PVI, PeerView Institute for Medical Education
Chair, Richard K. Bogan, MD, FCCP, FAASM, discusses sleep disorders in this CME/MOC activity titled “Navigating Narcolepsy in Family Practice: Patient-Centered Strategies to Optimize the Experience and Outcomes of Treatment.” For the full presentation, downloadable Practice Aids, and complete CME/MOC information, and to apply for credit, please visit us at https://bit.ly/48QOONd. CME/MOC credit will be available until April 9, 2025.
Navigating Narcolepsy in Family Practice: Patient-Centered Strategies to Opti...

Navigating Narcolepsy in Family Practice: Patient-Centered Strategies to Opti...PVI, PeerView Institute for Medical Education
Chair and Presenter, Jennifer Wargo, MD, MMSc, Charlotte E. Ariyan, MD, PhD, and Hussein Tawbi, MD, PhD, discuss melanoma in this CME/MOC/AAPA/IPCE activity titled “New Chapters in the Immunotherapy Story for Melanoma: Collaborative Care and Next Steps With Adjuvant and Neoadjuvant Therapy.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3T70bfi. CME/MOC/AAPA/IPCE credit will be available until April 21, 2025.New Chapters in the Immunotherapy Story for Melanoma: Collaborative Care and ...

New Chapters in the Immunotherapy Story for Melanoma: Collaborative Care and ...PVI, PeerView Institute for Medical Education
Chair and Presenters Laura S. Dominici, MD, FACS, Albert Henry Diehl, III, MD, FACS, and Jane L. Meisel, MD, discuss breast cancer in this CME/MOC/CC activity titled “Unraveling the Complex Choices in Early Breast Cancer: A Roadmap to Informed Multidisciplinary Decisions About Assessment and Treatment.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CC information, and to apply for credit, please visit us at https://bit.ly/42BZBZE. CME/MOC/CC credit will be available until April 21, 2025.Unraveling the Complex Choices in Early Breast Cancer: A Roadmap to Informed ...

Unraveling the Complex Choices in Early Breast Cancer: A Roadmap to Informed ...PVI, PeerView Institute for Medical Education
Chair, Meghan C. Thompson, MD, discusses chronic lymphocytic leukemia in this CME/MOC/NCPD/CPE/AAPA/IPCE activity titled “From Resistance to Resilience in R/R CLL: Sequencing Strategies for Achieving Effective Continuous Care.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3uoWOWG. CME/MOC/NCPD/CPE/AAPA/IPCE credit will be available until April 4, 2025.From Resistance to Resilience in R/R CLL: Sequencing Strategies for Achieving...

From Resistance to Resilience in R/R CLL: Sequencing Strategies for Achieving...PVI, PeerView Institute for Medical Education
Chair, James E. Galvin, MD, MPH, prepared useful Practice Aids pertaining to Alzheimer’s disease for this CME activity titled “Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating the New Diagnostic Tools and Disease-Modifying Therapies Into Your Clinical Practice.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/3Sk3DRJ. CME credit will be available until April 2, 2025.Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating ...

Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating ...PVI, PeerView Institute for Medical Education
Chair, James E. Galvin, MD, MPH, discusses Alzheimer’s disease in this CME activity titled “Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating the New Diagnostic Tools and Disease-Modifying Therapies Into Your Clinical Practice.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/3Sk3DRJ. CME credit will be available until April 2, 2025.Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating ...

Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating ...PVI, PeerView Institute for Medical Education
Chair and Presenter, Thomas G. Martin, III, MD, and Beth Faiman, PhD, MSN, APN-BC, AOCN, BMTCN, FAAN, FAPO, discuss multiple myeloma in this CME/MOC/NCPD/IPCE activity titled “The A-Team Against Relapsed/Refractory Myeloma: Community Strategies for Enhancing Outcomes With Potent CD38 Antibody Platforms.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/IPCE information, and to apply for credit, please visit us at https://bit.ly/3rUwBOx. CME/MOC/NCPD/IPCE credit will be available until November 29, 2024.The A-Team Against Relapsed/Refractory Myeloma: Community Strategies for Enha...

The A-Team Against Relapsed/Refractory Myeloma: Community Strategies for Enha...PVI, PeerView Institute for Medical Education
Chair and Moderator, Prof. Dr. med. Stephan Stilgenbauer, Matthew S. Davids, MD, MMSc, and Dr. Lydia Scarfò, MD, discuss chronic lymphocytic leukemia in this CME/MOC/CPD activity titled “Finite Therapy, Infinite Possibilities in CLL: Exploring the Rapid Emergence of Newer Time-Limited BTKi Combinations.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CPD information, and to apply for credit, please visit us at https://bit.ly/47sAiuM. CME/MOC/CPD credit will be available until April 18, 2025.Finite Therapy, Infinite Possibilities in CLL: Exploring the Rapid Emergence ...

Finite Therapy, Infinite Possibilities in CLL: Exploring the Rapid Emergence ...PVI, PeerView Institute for Medical Education
Mais conteúdo relacionado
Mais de PVI, PeerView Institute for Medical Education
Co-Chairs, Joseph K. Han, MD, and Seth J. Isaacs, MD, prepared useful Practice Aids pertaining to chronic rhinosinusitis with nasal polyps for this CME/MOC/CC/AAPA/IPCE activity titled “Biologics in CRSwNP: Putting a Paradigm Shift Into Practice.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3Tq6n1G. CME/MOC/CC/AAPA/IPCE credit will be available until May 6, 2025.Biologics in CRSwNP: Putting a Paradigm Shift Into Practice

Biologics in CRSwNP: Putting a Paradigm Shift Into PracticePVI, PeerView Institute for Medical Education
Co-Chairs, Joseph K. Han, MD, and Seth J. Isaacs, MD, discuss chronic rhinosinusitis with nasal polyps in this CME/MOC/CC/AAPA/IPCE activity titled “Biologics in CRSwNP: Putting a Paradigm Shift Into Practice.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3Tq6n1G. CME/MOC/CC/AAPA/IPCE credit will be available until May 6, 2025.Biologics in CRSwNP: Putting a Paradigm Shift Into Practice

Biologics in CRSwNP: Putting a Paradigm Shift Into PracticePVI, PeerView Institute for Medical Education
Co-Chairs, R. Donald Harvey, PharmD, BCOP, FCCP, FHOPA, FASCO, Zahra Mahmoudjafari, PharmD, MBA, BCOP, FHOPA, and James Davis, PharmD, BCOP, discuss multiple myeloma in this CME/CPE/IPCE activity titled “Prescriptions for Successful Myeloma Care: Pharmacy Strategies for Delivering Effective Therapy With Antibody Platforms.” For the full presentation, downloadable Practice Aids, and complete CME/CPE/IPCE information, and to apply for credit, please visit us at https://bit.ly/4aa0iMX. CME/CPE/IPCE credit will be available until May 2, 2025.Prescriptions for Successful Myeloma Care: Pharmacy Strategies for Delivering...

Prescriptions for Successful Myeloma Care: Pharmacy Strategies for Delivering...PVI, PeerView Institute for Medical Education
Co-Chairs, Carlos G. Romo, MD, and Aimee Sato, MD, discuss Neurofibromatosis in this CME/MOC activity titled “Precision & Progress Against NF1: Solutions for Better Outcomes With MEKi & Multimodal Care for NF1 pNF and Other Tumors.” For the full presentation, downloadable Practice Aids, and complete CME/MOC information, and to apply for credit, please visit us at https://bit.ly/3SZRz8p. CME/MOC credit will be available until May 2, 2025.Precision & Progress Against NF1: Solutions for Better Outcomes With MEKi & M...

Precision & Progress Against NF1: Solutions for Better Outcomes With MEKi & M...PVI, PeerView Institute for Medical Education
Chair and Presenters Kathleen N. Moore, MD, MS, Floor J. Backes, MD, and Bhavana Pothuri, MD, MS, prepared useful Practice Aids pertaining to endometrial cancer for this CME/MOC/NCPD/AAPA/IPCE activity titled “Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Potential of Immunotherapy, ADCs, PARP Inhibitors, and Other Emerging Treatment Strategies.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3SjJyuH. CME/MOC/NCPD/AAPA/IPCE credit will be available until April 17, 2025.Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...

Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...PVI, PeerView Institute for Medical Education
Chair and Presenters Kathleen N. Moore, MD, MS, Floor J. Backes, MD, and Bhavana Pothuri, MD, MS, discuss endometrial cancer in this CME/MOC/NCPD/AAPA/IPCE activity titled “Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Potential of Immunotherapy, ADCs, PARP Inhibitors, and Other Emerging Treatment Strategies.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3SjJyuH. CME/MOC/NCPD/AAPA/IPCE credit will be available until April 17, 2025.Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...

Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...PVI, PeerView Institute for Medical Education
Chair and Presenters Bradley J. Monk, MD, FACS, FACOG, Kathleen N. Moore, MD, MS, and Ana Oaknin, MD, PhD, discuss gynecologic cancers in this CME/MOC/NCPD/AAPA/IPCE activity titled “Advancing ADCs in Gynecologic Cancers: Expert Insights on Recent Evidence, Implementation Strategies, and Patient Care.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/4a56tly. CME/MOC/NCPD/AAPA/IPCE credit will be available until April 16, 2025.Advancing ADCs in Gynecologic Cancers: Expert Insights on Recent Evidence, Im...

Advancing ADCs in Gynecologic Cancers: Expert Insights on Recent Evidence, Im...PVI, PeerView Institute for Medical Education
Chair Lecia V. Sequist, MD, MPH, and Patrick Nana-Sinkam, MD, FCCP, prepared useful Practice Aids pertaining to lung cancer for this CME/MOC/AAPA/IPCE activity titled “Screening and Early Intervention as the Keys to Success in Lung Cancer: A Practical Approach to Implementing Lung Cancer Screening for High-Risk Individuals.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/46VvwoP. CME/MOC/AAPA/IPCE credit will be available until April 16, 2025.Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...

Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...PVI, PeerView Institute for Medical Education
Chair Lecia V. Sequist, MD, MPH, and Patrick Nana-Sinkam, MD, FCCP, discuss lung cancer screening in this CME/MOC/AAPA/IPCE activity titled “Screening and Early Intervention as the Keys to Success in Lung Cancer: A Practical Approach to Implementing Lung Cancer Screening for High-Risk Individuals.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/46VvwoP. CME/MOC/AAPA/IPCE credit will be available until April 16, 2025.Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...

Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...PVI, PeerView Institute for Medical Education
Chair and Presenter, Olalekan Oluwole, MBBS, MPH, Veronika Bachanova, MD, PhD, and David L. Porter, MD, prepared useful Practice Aids pertaining to CAR-T therapy for this CME/NCPD activity titled “Democratizing the CAR-T Experience: The Principles and Practice of Outpatient Cellular Therapy.” For the full presentation, downloadable Practice Aids, and complete CME/NCPD information, and to apply for credit, please visit us at https://bit.ly/3TfIABM. CME/NCPD credit will be available until April 15, 2025.Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...

Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...PVI, PeerView Institute for Medical Education
Chair and Presenter, Olalekan Oluwole, MBBS, MPH, Veronika Bachanova, MD, PhD, and David L. Porter, MD, discuss CAR-T therapy in this CME/NCPD activity titled “Democratizing the CAR-T Experience: The Principles and Practice of Outpatient Cellular Therapy.” For the full presentation, downloadable Practice Aids, and complete CME/NCPD information, and to apply for credit, please visit us at https://bit.ly/3TfIABM. CME/NCPD credit will be available until April 15, 2025.Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...

Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...PVI, PeerView Institute for Medical Education
Co-Chairs Lipika Goyal, MD, MPhil, and Riad Salem, MD, MBA, discuss HCC in this CME activity titled “The Convergence of Interventional Radiologists and Oncologists in HCC: Shared Decision-Making and Care Coordination at the Center of Personalized Care Across the Disease Continuum.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/48BAasz. CME credit will be available until April 26, 2025.The Convergence of Interventional Radiologists and Oncologists in HCC: Shared...

The Convergence of Interventional Radiologists and Oncologists in HCC: Shared...PVI, PeerView Institute for Medical Education
Chair, Richard K. Bogan, MD, FCCP, FAASM, discusses sleep disorders in this CME/MOC activity titled “Navigating Narcolepsy in Family Practice: Patient-Centered Strategies to Optimize the Experience and Outcomes of Treatment.” For the full presentation, downloadable Practice Aids, and complete CME/MOC information, and to apply for credit, please visit us at https://bit.ly/48QOONd. CME/MOC credit will be available until April 9, 2025.
Navigating Narcolepsy in Family Practice: Patient-Centered Strategies to Opti...

Navigating Narcolepsy in Family Practice: Patient-Centered Strategies to Opti...PVI, PeerView Institute for Medical Education
Chair and Presenter, Jennifer Wargo, MD, MMSc, Charlotte E. Ariyan, MD, PhD, and Hussein Tawbi, MD, PhD, discuss melanoma in this CME/MOC/AAPA/IPCE activity titled “New Chapters in the Immunotherapy Story for Melanoma: Collaborative Care and Next Steps With Adjuvant and Neoadjuvant Therapy.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3T70bfi. CME/MOC/AAPA/IPCE credit will be available until April 21, 2025.New Chapters in the Immunotherapy Story for Melanoma: Collaborative Care and ...

New Chapters in the Immunotherapy Story for Melanoma: Collaborative Care and ...PVI, PeerView Institute for Medical Education
Chair and Presenters Laura S. Dominici, MD, FACS, Albert Henry Diehl, III, MD, FACS, and Jane L. Meisel, MD, discuss breast cancer in this CME/MOC/CC activity titled “Unraveling the Complex Choices in Early Breast Cancer: A Roadmap to Informed Multidisciplinary Decisions About Assessment and Treatment.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CC information, and to apply for credit, please visit us at https://bit.ly/42BZBZE. CME/MOC/CC credit will be available until April 21, 2025.Unraveling the Complex Choices in Early Breast Cancer: A Roadmap to Informed ...

Unraveling the Complex Choices in Early Breast Cancer: A Roadmap to Informed ...PVI, PeerView Institute for Medical Education
Chair, Meghan C. Thompson, MD, discusses chronic lymphocytic leukemia in this CME/MOC/NCPD/CPE/AAPA/IPCE activity titled “From Resistance to Resilience in R/R CLL: Sequencing Strategies for Achieving Effective Continuous Care.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/CPE/AAPA/IPCE information, and to apply for credit, please visit us at https://bit.ly/3uoWOWG. CME/MOC/NCPD/CPE/AAPA/IPCE credit will be available until April 4, 2025.From Resistance to Resilience in R/R CLL: Sequencing Strategies for Achieving...

From Resistance to Resilience in R/R CLL: Sequencing Strategies for Achieving...PVI, PeerView Institute for Medical Education
Chair, James E. Galvin, MD, MPH, prepared useful Practice Aids pertaining to Alzheimer’s disease for this CME activity titled “Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating the New Diagnostic Tools and Disease-Modifying Therapies Into Your Clinical Practice.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/3Sk3DRJ. CME credit will be available until April 2, 2025.Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating ...

Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating ...PVI, PeerView Institute for Medical Education
Chair, James E. Galvin, MD, MPH, discusses Alzheimer’s disease in this CME activity titled “Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating the New Diagnostic Tools and Disease-Modifying Therapies Into Your Clinical Practice.” For the full presentation, downloadable Practice Aids, and complete CME information, and to apply for credit, please visit us at https://bit.ly/3Sk3DRJ. CME credit will be available until April 2, 2025.Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating ...

Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating ...PVI, PeerView Institute for Medical Education
Chair and Presenter, Thomas G. Martin, III, MD, and Beth Faiman, PhD, MSN, APN-BC, AOCN, BMTCN, FAAN, FAPO, discuss multiple myeloma in this CME/MOC/NCPD/IPCE activity titled “The A-Team Against Relapsed/Refractory Myeloma: Community Strategies for Enhancing Outcomes With Potent CD38 Antibody Platforms.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/NCPD/IPCE information, and to apply for credit, please visit us at https://bit.ly/3rUwBOx. CME/MOC/NCPD/IPCE credit will be available until November 29, 2024.The A-Team Against Relapsed/Refractory Myeloma: Community Strategies for Enha...

The A-Team Against Relapsed/Refractory Myeloma: Community Strategies for Enha...PVI, PeerView Institute for Medical Education
Chair and Moderator, Prof. Dr. med. Stephan Stilgenbauer, Matthew S. Davids, MD, MMSc, and Dr. Lydia Scarfò, MD, discuss chronic lymphocytic leukemia in this CME/MOC/CPD activity titled “Finite Therapy, Infinite Possibilities in CLL: Exploring the Rapid Emergence of Newer Time-Limited BTKi Combinations.” For the full presentation, downloadable Practice Aids, and complete CME/MOC/CPD information, and to apply for credit, please visit us at https://bit.ly/47sAiuM. CME/MOC/CPD credit will be available until April 18, 2025.Finite Therapy, Infinite Possibilities in CLL: Exploring the Rapid Emergence ...

Finite Therapy, Infinite Possibilities in CLL: Exploring the Rapid Emergence ...PVI, PeerView Institute for Medical Education
Mais de PVI, PeerView Institute for Medical Education (20)
Biologics in CRSwNP: Putting a Paradigm Shift Into Practice

Biologics in CRSwNP: Putting a Paradigm Shift Into Practice
Biologics in CRSwNP: Putting a Paradigm Shift Into Practice

Biologics in CRSwNP: Putting a Paradigm Shift Into Practice
Prescriptions for Successful Myeloma Care: Pharmacy Strategies for Delivering...

Prescriptions for Successful Myeloma Care: Pharmacy Strategies for Delivering...
Precision & Progress Against NF1: Solutions for Better Outcomes With MEKi & M...

Precision & Progress Against NF1: Solutions for Better Outcomes With MEKi & M...
Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...

Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...
Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...

Redefining Endometrial and Ovarian Carcinoma Care: Maximizing the Clinical Po...
Advancing ADCs in Gynecologic Cancers: Expert Insights on Recent Evidence, Im...

Advancing ADCs in Gynecologic Cancers: Expert Insights on Recent Evidence, Im...
Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...

Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...
Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...

Screening and Early Intervention as the Keys to Success in Lung Cancer: A Pra...
Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...

Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...
Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...

Democratizing the CAR-T Experience: The Principles and Practice of Outpatient...
The Convergence of Interventional Radiologists and Oncologists in HCC: Shared...

The Convergence of Interventional Radiologists and Oncologists in HCC: Shared...
Navigating Narcolepsy in Family Practice: Patient-Centered Strategies to Opti...

Navigating Narcolepsy in Family Practice: Patient-Centered Strategies to Opti...
New Chapters in the Immunotherapy Story for Melanoma: Collaborative Care and ...

New Chapters in the Immunotherapy Story for Melanoma: Collaborative Care and ...
Unraveling the Complex Choices in Early Breast Cancer: A Roadmap to Informed ...

Unraveling the Complex Choices in Early Breast Cancer: A Roadmap to Informed ...
From Resistance to Resilience in R/R CLL: Sequencing Strategies for Achieving...

From Resistance to Resilience in R/R CLL: Sequencing Strategies for Achieving...
Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating ...

Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating ...
Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating ...

Navigating Advances in Alzheimer’s Disease: An Expert Consult on Integrating ...
The A-Team Against Relapsed/Refractory Myeloma: Community Strategies for Enha...

The A-Team Against Relapsed/Refractory Myeloma: Community Strategies for Enha...
Finite Therapy, Infinite Possibilities in CLL: Exploring the Rapid Emergence ...

Finite Therapy, Infinite Possibilities in CLL: Exploring the Rapid Emergence ...
Último
Último (20)
8980367676 Call Girls In Ahmedabad Escort Service Available 24×7 In Ahmedabad

8980367676 Call Girls In Ahmedabad Escort Service Available 24×7 In Ahmedabad
Most Beautiful Call Girl in Bangalore Contact on Whatsapp

Most Beautiful Call Girl in Bangalore Contact on Whatsapp
9630942363 Genuine Call Girls In Ahmedabad Gujarat Call Girls Service

9630942363 Genuine Call Girls In Ahmedabad Gujarat Call Girls Service
Coimbatore Call Girls in Coimbatore 7427069034 genuine Escort Service Girl 10...

Coimbatore Call Girls in Coimbatore 7427069034 genuine Escort Service Girl 10...
Call Girls Service Jaipur {8445551418} ❤️VVIP BHAWNA Call Girl in Jaipur Raja...

Call Girls Service Jaipur {8445551418} ❤️VVIP BHAWNA Call Girl in Jaipur Raja...
Low Rate Call Girls Bangalore {7304373326} ❤️VVIP NISHA Call Girls in Bangalo...

Low Rate Call Girls Bangalore {7304373326} ❤️VVIP NISHA Call Girls in Bangalo...
Call Girls Amritsar Just Call 8250077686 Top Class Call Girl Service Available

Call Girls Amritsar Just Call 8250077686 Top Class Call Girl Service Available
Russian Call Girls Service Jaipur {8445551418} ❤️PALLAVI VIP Jaipur Call Gir...

Russian Call Girls Service Jaipur {8445551418} ❤️PALLAVI VIP Jaipur Call Gir...
Dehradun Call Girls Service {8854095900} ❤️VVIP ROCKY Call Girl in Dehradun U...

Dehradun Call Girls Service {8854095900} ❤️VVIP ROCKY Call Girl in Dehradun U...
Independent Call Girls In Jaipur { 8445551418 } ✔ ANIKA MEHTA ✔ Get High Prof...

Independent Call Girls In Jaipur { 8445551418 } ✔ ANIKA MEHTA ✔ Get High Prof...
Russian Call Girls Lucknow Just Call 👉👉7877925207 Top Class Call Girl Service...

Russian Call Girls Lucknow Just Call 👉👉7877925207 Top Class Call Girl Service...
Call Girls Jaipur Just Call 9521753030 Top Class Call Girl Service Available

Call Girls Jaipur Just Call 9521753030 Top Class Call Girl Service Available
Call Girls in Delhi Triveni Complex Escort Service(🔝))/WhatsApp 97111⇛47426

Call Girls in Delhi Triveni Complex Escort Service(🔝))/WhatsApp 97111⇛47426
Call Girls Coimbatore Just Call 8250077686 Top Class Call Girl Service Available

Call Girls Coimbatore Just Call 8250077686 Top Class Call Girl Service Available
Call Girls Varanasi Just Call 8250077686 Top Class Call Girl Service Available

Call Girls Varanasi Just Call 8250077686 Top Class Call Girl Service Available
Call Girls Rishikesh Just Call 8250077686 Top Class Call Girl Service Available

Call Girls Rishikesh Just Call 8250077686 Top Class Call Girl Service Available
Call Girl In Pune 👉 Just CALL ME: 9352988975 💋 Call Out Call Both With High p...

Call Girl In Pune 👉 Just CALL ME: 9352988975 💋 Call Out Call Both With High p...
Call Girls Kolkata Kalikapur 💯Call Us 🔝 8005736733 🔝 💃 Top Class Call Girl Se...

Call Girls Kolkata Kalikapur 💯Call Us 🔝 8005736733 🔝 💃 Top Class Call Girl Se...
Andheri East ^ (Genuine) Escort Service Mumbai ₹7.5k Pick Up & Drop With Cash...

Andheri East ^ (Genuine) Escort Service Mumbai ₹7.5k Pick Up & Drop With Cash...
Changing the Paradigm of CMV Management: New Science and More Choices for Challenging Cases in the HCT Setting
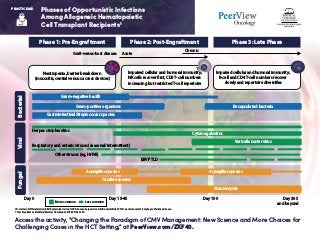
- 1. Phases of Opportunistic Infections Among Allogeneic Hematopoietic Cell Transplant Recipients1 Access the activity, “Changing the Paradigm of CMV Management: New Science and More Choices for Challenging Cases in the HCT Setting,” at PeerView.com/ZXF40. PRACTICE AID CD: cluster of differentiation; EBV: Epstein-Barr virus; HHV6; human herpesvirus 6; NK: natural killer; PTLD: post-transplant lymphoproliferative disease. 1. Tomblyn M et al. Biol Blood Marrow Transplant. 2009;15:1143-1238. Phase 1: Pre-Engraftment BacterialViralFungal Phase 2: Post-Engraftment Phase 3: Late Phase Neutropenia, barrier breakdown (mucositis, central venous access devices) Gram-negative bacilli Encapsulated bacteria Varicella zoster virus Aspergillus speciesAspergillus species Candida species Pneumocystis Gastrointestinal Streptococcus species Gram-positive organisms Impaired cellular and humoral immunity; NK cells recover first, CD8 T-cell numbers increasing but restricted T-cell repertoire Impaired cellular and humoral immunity; B-cell and CD4 T-cell numbers recover slowly and repertoire diversifies Graft-versus-host disease: Day 0 Day 15-45 Day 100 More common Day 365 and beyond Acute Chronic Less common Herpes simplex virus Respiratory and enteric viruses (seasonal/intermittent) Other viruses (eg, HHV6) Cytomegalovirus EBV PTLD
- 2. PRACTICE AID Access the activity, “Changing the Paradigm of CMV Management: New Science and More Choices for Challenging Cases in the HCT Setting,” at PeerView.com/ZXF40. Preventing CMV infection and Disease in HCT Recipients1 Comparison of Current CMV Prevention Strategies The Newest FDA-Approved Drug for CMV Prophylaxis in Allogeneic HCT Recipients: Key Characteristics of Letermovir Prophylaxis Antivirals administered to all at-risk patients for a defined period of time following transplantation CMV DNA test (at least once weekly) Antiviral therapy started when viral load exceeds a certain threshold Effectively prevents early CMV reactivation High rates of medication side effects Increased drug cost Risk for delayed-onset CMV disease Preemptive Therapy 3,4-dihydro-quinazoline-4-yl-acetic acid derivative Only active against CMV (no activity against HSV) Uncommon, mainly GI (gastritis, nausea), dyspnea, hepatitis 93% in feces, mostly as unchanged drug CMV prophylaxis in CMV-seropositive HCT recipients Inhibits terminase complex subunit pUL56 94% healthy individuals; 35% in HCT (increased to 85% with cyclosporine) Reduces exposure to voriconazole; increases exposure to tacrolimus, cyclosporine, midazolam; letermovir exposure increased with cyclosporine 480 mg daily (240 mg if administered with cyclosporine) for prophylaxis in HCT; no dose adjustment for renal dysfunction Use of letermovir for CMV prophylaxis in CMV-seronegative kidney transplant recipients Principle Advantages Disadvantages Reduced medication cost Lower risk of drug toxicity Allows immune reconstitution DoesnotpreventearlyCMVreactivation Escape CMV infections (not detected by weekly CMV NAT) Burden, logistics, cost of weekly surveillance labs Molecule Mechanism of action Spectrum of activity Bioavailability Excretion Dosing Side effects Drug interactions Current FDA approval indication Ongoing trials
- 3. PRACTICE AID Access the activity, “Changing the Paradigm of CMV Management: New Science and More Choices for Challenging Cases in the HCT Setting,” at PeerView.com/ZXF40. CMV: cytomegalovirus; GCV: ganciclovir; HCT: hematopoietic stem cell transplantation; NAT: nucleic acid testing; QTc: corrected QT interval; SOT: solid organ transplantation; VGCV: valganciclovir. 1. El Helou G, Razonable RR. Infect Drug Resist. 2019;12:1481-1491. Mechanism of action • 2ʹ-deoxyguanosine analog • Competitive binding to UL54 • DNA polymerase • Needs phosphorylation by CMV (UL97 encoded) and host kinases • Virostatic agent • Pyrophosphate analog • Noncompetitive inhibitor of many RNA and DNA polymerases (UL54 DNA polymerase in CMV) • Virostatic agent • Acyclic monophosphate deoxycytidine analog • Competitive substrate of UL54 DNA polymerase leads to inhibition of viral DNA synthesis through incorporation into growing viral DNA chain • Virostatic agent • Inhibits viral terminase complex, encoded by genes UL56, UL51, and UL89 • Virostatic agent Indications/ uses • CMV retinitis • CMV prophylaxis SOT Non-FDA uses • CMV disease • CMV preemptive strategy • CMV prophylaxis in HCT • CMV retinitis Non-FDA uses • Second line for GCV- resistant CMV disease therapy, prophylaxis, or preemptive therapy • CMV retinitis Non-FDA uses • Second line for GCV- resistant CMV disease therapy, prophylaxis, or preemptive therapy • CMV prophylaxis in CMV-seropositive HCT recipients Formulations • GCV IV only • VGCV oral • IV only • IV only • Lipid conjugate not yet approved (brincidofovir) • IV and oral Adverse effects • Pancytopenia and myelosuppression (leukopenia/ neutropenia++) • Renal injury • Diarrhea • Less common: pruritus, nausea, fever, torsade de pointe • Renal injury • Electrolytes wasting • Neutropenia • Less common: headache, diarrhea, fever, QTc prolongation • Renal injury • Proteinuria • Neutropenia • Ocular toxicity (iritis, uveitis, amblyopia) • Less common: headaches, shivering, rash, alopecia, dyspnea • Uncommon, mainly GI (gastritis, nausea), dyspnea, hepatitis Resistance mechanism • Mutations in UL97 gene prevent activation of drug • Mutations in UL54 gene prevent binding to DNA polymerase (may confer cross-resistance with all DNA-polymerase active antivirals) • Mutations in UL54 gene prevent binding to DNA polymerase (may confer cross-resistance with all DNA-polymerase active antivirals) • Mutations in UL54 gene prevent binding to DNA polymerase (may confer cross-resistance with all DNA-polymerase active antivirals) • Mutations in UL56 gene • Less commonly, mutations in UL51 or UL89 genes Characteristics of Antiviral Drugs Approved for CMV Ganciclovir and Valganciclovir Foscarnet Cidofovir Letermovir Preventing CMV infection and Disease in HCT Recipients1
- 4. Access the activity, “Changing the Paradigm of CMV Management: New Science and More Choices for Challenging Cases in the HCT Setting,” at PeerView.com/ZXF40. PRACTICE AID A Closer Look at Resistant and Refractory CMV Infection and Disease in HCT Recipients1,2 Risk Factors for CMV Resistance in HCT Recipientsa Summary of the Definitions of Refractory CMV Infection and Disease and Antiviral Drug Resistance for Use in Clinical Trials Host factors Host factors Viral factors • Prolonged antiviral CMV drug exposure (>3 mo) • Previous antiviral CMV drug exposure • Recurrent CMV infection • Inadequate antiviral CMV drug absorption and bioavailability • Inadequate antiviral CMV oral prodrug conversion • Variation in antiviral CMV drug clearance • Subtherapeutic antiviral CMV drug level • Poor patient compliance with antiviral drug regimen • T-cell depletion • Haploidentical, allogeneic, or cord blood HCT • Delayed immune reconstitution • CMV-seropositive recipient and CMV-seronegative donor • Treatment with antithymocyte antibodies • Active GVHD • Young age • Congenital immunodeficiency syndromes Refractory CMV infection Probable refractory CMV infection Refractory CMV end-organ disease Probable refractory CMV end-organ disease Antiviral drug resistance • CMV viral load rise while receiving treatment (after >2 wk of adequate dosing) • Failure of CMV viral load to fall despite appropriate treatment • Rise in CMV viral load after initial decline while receiving appropriate treatment • Intermittent low-level CMV viremia • High CMV viral loads Host factors CMV viremia that increasesb after at least 2 wk of appropriately dosed antiviral therapy Worsening in signs and symptoms or progression into end-organ disease after at least 2 wk of appropriately dosed antiviral therapy Viral genetic alteration that decreases susceptibility to one or more antiviral drugsd Persistent viral loadc after at least 2 wk of appropriately dosed antiviral therapy Lack of improvement in signs and symptoms after at least 2 wk of appropriately dosed antiviral drugs
- 5. Access the activity, “Changing the Paradigm of CMV Management: New Science and More Choices for Challenging Cases in the HCT Setting,” at PeerView.com/ZXF40. PRACTICE AID A Closer Look at Resistant and Refractory CMV Infection and Disease in HCT Recipients1,2 a Most of the risk factors for CMV resistance pertain to solid organ transplant recipients as well, in addition to graft rejection (instead of GVHD) and CMV-seropositive donor and CMV-seronegative recipient. b More than 1 log10 increase in CMV DNA levels in blood or serum and determined by log10 change from the peak viral load within the first week to the peak viral load at ≥2 weeks as measured in the same laboratory with the same assay. c CMV viral load at the same level or higher than the peak viral load within 1 week but <1 log10 increase in CMV DNA titers done in the same laboratory and with the same assay. d Known examples involve genes involved in antiviral drug anabolism (eg, UL97-mediated phosphorylation of ganciclovir), the antiviral drug target (eg, UL54, UL97, UL56/89/51), or compensation for antiviral inhibition of biological function (eg, UL27). CMV: cytomegalovirus; GVHD: graft-vs-host disease; HCT: hematopoietic cell transplantation; SOT: solid organ transplantation. 1. Chemaly RF et al. Clin Infect Dis. 2019;68:1420-1426. 2. Papanicolaou GA et al. Clin Infect Dis. 2019;68:1255-1264. Maribavir for Refractory or Resistant CMV Infections Cl N N N H HO O O O H H Cl A Phase 3, Multicenter, Randomized, Open-Label, Active-Controlled Study to Assess the Efficacy and Safety of Maribavir Treatment Compared to Investigator-Assigned Treatment in Transplant Recipients With Cytomegalovirus (CMV) Infections That Are Refractory or Resistant to Treatment With Ganciclovir, Valganciclovir, Foscarnet, or Cidofovir • Clinicaltrials.gov/NCT02931539 • Status: recruiting HCT + SOT Side effects: dysgeusia and nausea, vomiting Phase 3 trial Completed phase 3 trial Within 6 weeks, 67% of patients had undetectable plasma CMV DNA Refractory or resistant CMV infection
