Heat Capacity Specific heat-capacity (1)
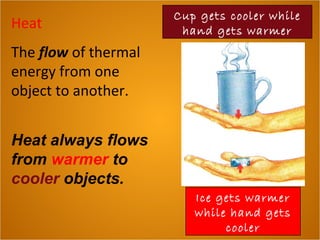
- 1. Heat The flow of thermal energy from one object to another. Heat always flows from warmer to cooler objects. Ice gets warmer while hand gets cooler Cup gets cooler while hand gets warmer
- 2. Imagine…Imagine… A hot day in your back yard is aA hot day in your back yard is a Piece of SteelPiece of Steel and aand a wood Blockwood Block.. Would you want to stick your hand to theWould you want to stick your hand to the wood or touch the steel?wood or touch the steel? SteelSteelSteelSteel WoodWood You would want to touch the wood becauseYou would want to touch the wood because
- 3. Things heat up or cool down at different rates. Land heats up and cools down faster than water, This is why land heats up quickly during the day and cools quickly at night and why water takes longer
- 4. Thermal (heat) capacity What requires more energy to heat up by 1o C? Different material heat up at different rate Different material store different amount of heat energy!!!
- 5. Heat Capacity The quantity of heat required to raise the temperature of any mass of a substance by one degree Celsius (or Kelvin) The unit is (J/ o C)
- 6. Equation: q = C x ΔT • q = heat transferred (in joules) • C = specific heat capacity • ΔT = change in temperature ΔT = Tfinal - Tinitial
- 7. Thermal (heat) capacity What requires more energy to heat up by 1o C? 1 kg water 1 kg aluminium1 kg aluminium 4200 joules of energy 900 joules of energy Water must be supplied with nearly five times as much energy as aluminium for the same rise in temperature. It’s all to do with the SPECIFIC HEAT CAPACITY of the material
- 8. You will possibly have noticed that it is easier to warm up a saucepan full of oil than it is to warm up one full of water. Specific Heat Capacity can be thought of as a measure of how much heat energy is needed to warm the substance up.
- 9. Things heat up or cool down at different rates. Land heats up and cools down faster than water, C water = 4184 J / kg C (“holds” its heat) C sand = 664 J / kg C (less E to change) This is why land heats up quickly during the day and cools quickly at night and why water takes longer
- 10. Specific Heat Capacity The quantity of heat required to raise the temperature of 1 kilo gram of a substance by one degree Celsius (or Kelvin) The unit is (J/kg o C)
- 11. Equation: q = m x C x ΔT • q = heat transferred (in joules) • m = mass (in Kilo grams) • C = specific heat capacity • ΔT = change in temperature ΔT = Tfinal - Tinitial
- 12. • The next table shows how much energy it takes to heat up some different substances. • The small values show that not a lot ofThe small values show that not a lot of energy is needed to produce aenergy is needed to produce a temperature change, whereas the largetemperature change, whereas the large values indicate a lot more energy isvalues indicate a lot more energy is needed.needed.
- 13. • Approximate values in J / kg °C of the Specific Heat Capacities of some substances are: Air 1000 Lead 125 Aluminium 900 Mercury 14 Asbestos 840 Nylon 1700 Brass 400 Paraffin 2100 Brick 750 Platinum 135 Concrete 3300 Polythene 2200 Cork 2000 Copper 390 Glass 600 Rubber 1600 Gold 130 Silver 235 Ice 2100 Steel 450 Iron 500 Water 4200
- 14. An example of a calculation using the specific heat capacity equation: How much energy would be needed to heat 450 grams of copper metal from a temperature of 25.0 ºC to a temperature of 75.0ºC? (The specific heat of copper is 385 J/Kg ºC.)