structure of atom
•Transferir como DOCX, PDF•
1 gostou•202 visualizações
structure of atom
Denunciar
Compartilhar
Denunciar
Compartilhar
Recomendados
Mais conteúdo relacionado
Mais procurados
Mais procurados (20)
ppt on Elementary Particles By Jyotibhooshan chaturvedi

ppt on Elementary Particles By Jyotibhooshan chaturvedi
Semelhante a structure of atom
Semelhante a structure of atom (20)
Structure of atom- discovery of proton, electron & neutron

Structure of atom- discovery of proton, electron & neutron
Mais de MISSRITIMABIOLOGYEXP
Mais de MISSRITIMABIOLOGYEXP (20)
PART 1 - CHAPTER 1 - CELL THE FUNDAMENTAL UNIT OF LIFE

PART 1 - CHAPTER 1 - CELL THE FUNDAMENTAL UNIT OF LIFE
CHAPTER 1 CROP PRODUCTION AND MANAGEMENT CLASS 8TH

CHAPTER 1 CROP PRODUCTION AND MANAGEMENT CLASS 8TH
HOW TO OBTAIN PURE COPPER SULPHATE FROM AN IMPURE SAMPLE

HOW TO OBTAIN PURE COPPER SULPHATE FROM AN IMPURE SAMPLE
HOW CAN WE OBTAIN COLOURED COMPONENT FROM BLUE OR BLACK INK?

HOW CAN WE OBTAIN COLOURED COMPONENT FROM BLUE OR BLACK INK?
Último
Último (20)
Locating and isolating a gene, FISH, GISH, Chromosome walking and jumping, te...

Locating and isolating a gene, FISH, GISH, Chromosome walking and jumping, te...
Vip profile Call Girls In Lonavala 9748763073 For Genuine Sex Service At Just...

Vip profile Call Girls In Lonavala 9748763073 For Genuine Sex Service At Just...
9654467111 Call Girls In Raj Nagar Delhi Short 1500 Night 6000

9654467111 Call Girls In Raj Nagar Delhi Short 1500 Night 6000
Module for Grade 9 for Asynchronous/Distance learning

Module for Grade 9 for Asynchronous/Distance learning
Human & Veterinary Respiratory Physilogy_DR.E.Muralinath_Associate Professor....

Human & Veterinary Respiratory Physilogy_DR.E.Muralinath_Associate Professor....
SAMASTIPUR CALL GIRL 7857803690 LOW PRICE ESCORT SERVICE

SAMASTIPUR CALL GIRL 7857803690 LOW PRICE ESCORT SERVICE
development of diagnostic enzyme assay to detect leuser virus

development of diagnostic enzyme assay to detect leuser virus
Asymmetry in the atmosphere of the ultra-hot Jupiter WASP-76 b

Asymmetry in the atmosphere of the ultra-hot Jupiter WASP-76 b
pumpkin fruit fly, water melon fruit fly, cucumber fruit fly

pumpkin fruit fly, water melon fruit fly, cucumber fruit fly
Biogenic Sulfur Gases as Biosignatures on Temperate Sub-Neptune Waterworlds

Biogenic Sulfur Gases as Biosignatures on Temperate Sub-Neptune Waterworlds
Justdial Call Girls In Indirapuram, Ghaziabad, 8800357707 Escorts Service

Justdial Call Girls In Indirapuram, Ghaziabad, 8800357707 Escorts Service
Thyroid Physiology_Dr.E. Muralinath_ Associate Professor

Thyroid Physiology_Dr.E. Muralinath_ Associate Professor
High Class Escorts in Hyderabad ₹7.5k Pick Up & Drop With Cash Payment 969456...

High Class Escorts in Hyderabad ₹7.5k Pick Up & Drop With Cash Payment 969456...
structure of atom
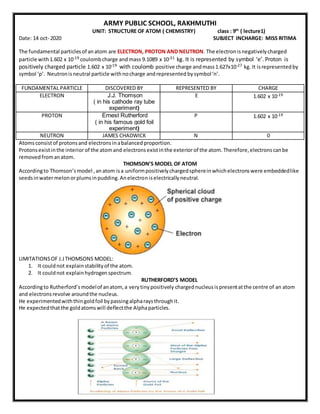
- 1. ARMY PUBLIC SCHOOL, RAKHMUTHI UNIT: STRUCTURE OF ATOM ( CHEMISTRY) class : 9th ( lecture1) Date: 14 oct- 2020 SUBJECT INCHARGE: MISS RITIMA The fundamental particlesof anatom are ELECTRON, PROTON AND NEUTRON. The electronisnegativelycharged particle with1.602 x 10-19 coulombcharge andmass 9.1089 x 10-31 kg. It is represented by symbol ‘e’. Proton is positively charged particle 1.602 x 10-19 with coulomb positive charge andmass1.627x10-27 kg. It isrepresentedby symbol ‘p’. Neutronisneutral particle withnocharge andrepresented bysymbol‘n’. FUNDAMENTAL PARTICLE DISCOVERED BY REPRESENTED BY CHARGE ELECTRON J.J. Thomson ( in his cathode ray tube experiment) E 1.602 x 10-19 PROTON Ernest Rutherford ( in his famous gold foil experiment) P 1.602 x 10-19 NEUTRON JAMES CHADWICK N 0 Atomsconsistof protonsand electronsinabalancedproportion. Protonsexistinthe interior of the atomand electronsexistinthe exterior of the atom.Therefore,electronscanbe removedfromanatom. THOMSON’S MODEL OF ATOM Accordingto Thomson’s model ,anatom isa uniformpositivelychargedsphereinwhichelectronswere embeddedlike seedsinwatermelonorplumsinpudding.Anelectroniselectricallyneutral. LIMITATIONSOF J.JTHOMSONS MODEL: 1. It couldnot explainstabilityof the atom. 2. It couldnot explainhydrogenspectrum. RUTHERFORD’S MODEL Accordingto Rutherford’smodelof anatom,a verytinypositively chargednucleusispresentatthe centre of an atom and electronsrevolve aroundthe nucleus. He experimentedwiththingoldfoil bypassingalpharaysthroughit. He expectedthatthe goldatomswill deflectthe Alphaparticles.
- 2. Observations Inferences Alpha particles which had high speed moved straight through the gold foil Atom contains a lot of empty space Some particles got diverted a by slide angles Positive charges in the atom are not occupying much of its space Only one out of 12000 particles bounced back The positive charges are concentrated over a particular area of the atom. LIMITATIONS OF RUTHERFORD’S MODEL: 1. It could not explain stability of the atom. 2. It couldnot explainhydrogenspectrum. BOHR’S MODEL OF ATOM The main pointsof Bohr’s model of atomare: 1. An atomhas three typesof particlescalledasfundamentalparticles.These are electrons,Protonsandneutrons. 2. The protonsand neutronsare presentinthe centre.The electronsare presentaroundthe nucleus. 3. An electroniselectricallyneutral asthe numberof protonsisequal tothe numberof electrons. 4. The electronsare revolvingaroundthe nucleusinfixedpathwhichare calledas ENERGYLEVELS OR ENERGY SHELLS OR ORBITS. The shellsare countedintwoways 1 , 2 , 3 , 4 , 5 , 6 or k , L , M , N , O , P. The countingis fromcentre to outwards. 5. The variousenergylevelsare arrangedin orderof increasingenergy. The orderof energyis: 1 < 2 < 3 < 4 < 5 < 6 or k < L < M < M < N < O < P 6. The energyof electroninan atom is quantized. 7. There isno change in the energyof the electronsaslong as they keeponrevolvinginthe same energylevel. 8. The angular momentumof anelectroninan atomis quantized. 9. The change inenergytakesplace whenanelectronjumps fromone energy level toanother. If electronsgainenergyfromoutside itjumpsfromlowerenergylevel tothe higherenergylevel. If electron jumpsfromhigherenergylevel tolowerenergy level, itlosesenergy.
- 3. READ AND REREAD: 1. Name the sub- atomicparticlesof an atom. Ans: Electron, Protonand Neutron. 2. On the basesof Rutherfordmodel whichsubatomicparticle ispresentinthe modecule of anatom? Ans: Proton 3. State three waysinwhichprotonsdifferfrom electron. PROTONS ELECTROS It has one unitof positive charge. It has one unitof negative charge.I It has massof 1.6 x 10-24 It has massof 1.6 x 10-28 It ispresentinthe nucleusof atom. It ispresentinthe extranuclearpartof an atom. 4. The fundamental particlespresentinatomwhichare equal innumberare: a. Protonand electron b. Neutronand electron c. Protonand neutron d. Protonsand positrons 5. A protonisidentical with: a. An atomof hydrogen b. A molecule of hydrogen c. The nucleusof helium d. The nucleusof hydrogenatom
