PPAR alpha poster
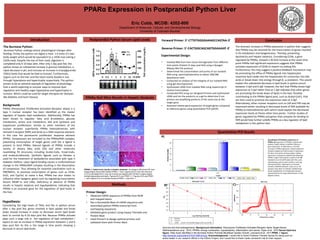
- 1. PPARα Expression in Postprandial Python Liver Introduction Eric Cobb, MCDB: 4202-800 Background: PPARα (Peroxisome Proliferator-Activated Receptor ahlpa) is a type II nuclear receptor has been identified as the master regulator of hepatic lipid metabolism. Additionally, PPARα has been shown to regulate: fatty acid β-oxidation, glucose metabolism, amino acid metabolism, bile acid synthesis and hepatocyte proliferation. Similar to other members of the nuclear receptor superfamily PPARα heterodimerizes with retinoid X receptor (RXR) and binds to a DNA response element, in this case the peroxisome proliferator response element (PPRE). Corepressors are recruited to the PPARα/RXR complex, preventing transcription of target genes until the a ligand is present to bind PPARα. Natural ligands of PPARα include a variety of dietary fatty acids (FA) and other molecules resembling FA structures including Acetyl-CoAs, Enoyl-CoAs, and endocannabinoids. Synthetic ligands such as fibrates is used for the treatment of dyslipidemia associated with type II diabetes mellitus. Upon ligand binding causes a conformational change in the PPARα/RXR complex resulting in the dissociation of corepressor. Thus allowing for essential coactivators such as PBP/MED1, to promote transcription of genes such as CD36, Scd1, and Cyp7a1 to name a few. PPARα has also shown to influence other lipogenic genes such by regulating transcription factors SREBP-1c and LXRα. Deficiency or absence of PPARα results in hepatic steatosis and hypolipedemia, indicating that PPARα is an essential gene for the regulation of lipid levels in the liver. Hypothesis: Considering the high levels of TAGs and FAs in python serum after 1 day post fed, genes involved in lipid uptake and break down should increase in order to decrease serum lipid levels back to normal by 6-10 days post fed. Because PPARα activate plays such a large role in the regulation of lipid metabolism I expect to see an increase in PPARα expression between 1 and 6 days post fed. As this is the range in time points showing a decrease in serum lipid levels. Department of Molecular, Cellular, and Developmental Biology University of Colorado-Boulder Quantitative PCR Results The Burmese Python: Burmese Python undergo drastic physiological changes after feeding. Firstly, the python can digest a meal 1.6 times it’s own body weight which would be equivalent to a 145lb man eating a 232lb meal. Despite the size of their meal, digestion is completed only 6-10 days later. After only 1 day post fed, the python shows an substantial increase in glucose metabolism, a rapid decrease in pH, and increase an increase in triacylglyceride (TAGs) levels that would be fatal to humans. Furthermore, organs such as the liver and the heart nearly double in size through hyperplasia and hypertrophy respectively. The python proves to be an extreme example of regulation of physiology that is worth exploring to uncover ways to improve lipid regulation and healthy organ hyperplasia and hypertrophy in humans. Which could be used for target therapies for diseases like diabetes and heart disease. Postprandial Python Serum Lipid LevelsIntroduction PPARα Null Mice Results in Hepatic Steatosis PPARα -/- fasted mice results in hepatic steatosis: (A) Image (Left) shows WT liver, image (Right) shows 66hr fasted PPARα -/- liver. Figures (B,D,F) are Fed mice and (C,E,G) are fasted 48 hr mice, all of which are stained with Oil Red O-stains (stains neutral triglycerides and lipids. Figures (B,C) are PPAR +/+, Figures (D,E) are PPAR - /-, and Figures (F,G) is a double knockout PPAR -/- and AOX -/-. Methods Primer Design: • Obtained mRNA sequence of PPARα from NCBI and mapped exons • Ran a Nucleotide Blast of mRNA sequence and assembled python PPARα transcript from overlapping sequences • Validated gene product using Expasy Translate and Protein Blast • Used Primer3 to design optimal primers and validated them with Primer Blast Forward Primer: 5’-CTTGTGGGGAAAGCCAGTAA-3’ Reverse Primer: 5’-CACTGGCAGCAGTGGAAAAT-3’ Experimental Design: • Isolated RNA from liver tissue homogenates from different time points (Fasted-15 days post fed) using a Quiagen RNeasy Mini Kit protocol • Determined the concentration and purity of our isolated RNA using spectrophotometry to obtain 260/280 absorbance ratio • Conducted an analysis of the integrity of our isolated RNA using gel electrophoresis • Synthesized cDNA from isolated RNA using Superscript III reverse transcriptase • Conducted PCR using our designed Primers and synthesized cDNA and ran the products on a gel to determine if our primers are amplifying products of the same size as the target gene • Assessed relative gene expression of target genes compared to reference genes using quantitative PCR (qPCR) Conclusion Quantitative PCR Relative Expression of PPARα: A) Expression of PPARα in python liver (Fasted-15dpf) relative to GAPDH reference gene expression. At 3dpf there is a 52 fold increase in PPARα expression. Fasted expression showed roughly one fold increase in expression. All other time points all show higher relative expression compared to GAPDH indicating that PPARα is high in abundance in python liver. B) Standard Curve for qPCR plate with the y-axis representing cycle number and the x-axis Log Starting Quantity. Target gene of unknown samples amplified between cycles 16-21. All samples were done in triplicate. C) Melt Peak shows amplification of a single gene product represented by the single peak between 75-80 degrees Celsius. A) B) C) The dramatic increase in PPARα expression in python liver suggests that PPARα may be essential for the transcription of genes involved in FA metabolism and lipogenenesis. Thereby, preventing lipotoxicity and hepatic steatosis. Considering CD36, a gene regulated by PPARα, showed a 36 fold increase at the same time point PPARα had significant expression suggests that PPARα activates expression of CD36 to import circulating FAs. Furthermore, this may suggest a positive feedback mechanism may be promoting the efflux of PPARα ligands into hepatocytes maximize lipid intake into the hepatocytes for conversion into bile acids or break down into energy through β, ω-oxidation. This would explain the substantial decrease in lipid levels in python serum between 3 dpf and 6 dpf. However, the fact that PPARα shows high expression at 3 dpf rather than at 1 dpf indicates that other genes are promoting the break down of lipids in the liver. Possibly contributing to the PPARα ligand pool, such as Acetyl-CoA’s, that are then used to promote the expression seen at 3 dpf. Alternatively, other nuclear receptors such as LXR and FXR may be expressed earlier resulting in decreased levels of RXR available for PPARα to heterodimerize with which could explain the decreased expression levels at theses other time points. Further studies of genes regulated by PPARα and genes that compete for binding to RXR would help further solidify PPARα as a key regulator of lipid metabolism in the python liver. Sources and Acknowledgements: Background information: Peroxisome Proliferator-Activated Receptor Alpha Target Genes, Rakhshandehroo et al., 2010; PPARα: energy combustion, hypolipidemia, inflammation and cancer, Pyper et al., 2010 Serum lipid leve figure: Fatty Acids Identified in the Burmese Python Promote Beneficial Cardiac Growth, Leinwand et al., 2011:PPAR -/- figure: http://www.jbc.org/content/275/37/28918.long. Acknowledgments: I would like to thank Professor Pamela Harvey for being such an active leader in our research efforts in the Python Project, and I would like to thank Leslie Leinwand’s lab for their support.