Origin and characteristics of salt-affected soils
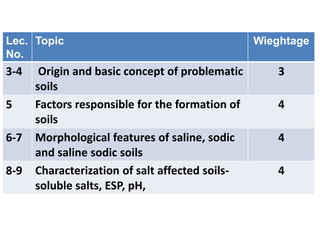
- 1. Lec. No. Topic Wieghtage 3-4 Origin and basic concept of problematic soils 3 5 Factors responsible for the formation of soils 4 6-7 Morphological features of saline, sodic and saline sodic soils 4 8-9 Characterization of salt affected soils- soluble salts, ESP, pH, 4
- 2. Types of soil degradation Water Erosion Sheet erosion Rill erosion Gully erosion Wind Erosion Chemical Degradation Acid soil Saline soil Saline- sodic soil Sodic soil Acid- sulphate soil Coastal saline soil Physical degradation Water logging (Submerged ) Surface ponding Subsurface water logging Vegetation degradation Land sliding Soil fertility decline Other degradation Mining Industria l waste appli Snow covered area
- 3. Causes of DegradationDeforestation Overgrazing Agricultural Practices Over- exploitation of vegetative cover Industrial activity Land use conversion Soil nutrient mining Inappropriate irrigation
- 4. Physical processes Structural decline Compaction- Heavy implements Erosion: Wind/water High soil temperature Leaching Chemical Processes Nutrient imbalance/ Fertility depletion Salinization/ Calcareous soils (Pri/Sec) Sodicity Acidification Marshy lands Submerged soils Acid sulphate soils Land sliding Biological Processes Soil biodiversity decrease Decrease in OM Intensive cultivation Soil Pollution Deforestation Forest fire Factors and Processes of Soil Degradation
- 5. NEGATIVE POSITIVE Soil erosion Conservation tillage Nutrient runoff loss Crop rotation Water logging Improved drainage Desertification Residue management Acidification Water conservation Compaction Terracing Crusting Contour farming Organic matter loss Efficient nutrient use Salinization Green manuring Nutrient depletion by leaching Improved nutrient cycling Toxicant acculmulation Soil climate Calcareousness Mulching Sodicity INM Acid sulphate soils
- 6. Classification of problem soils based on properties Physical characteristics Eroded soils Sloping lands Ravine lands Highly permeable coarse textured soils Crusting soils Red chalka soils Peat soils Submerged soils/Marshy land Slowly permeable heavy textured soils Chemical Characteristics Acid soils Acid sulphate soils Calcareous soils Salt affected soils
- 7. Classification – Problem Soils Salt Affected Soil Saline soils Saline-sodic soils Sodic soils Other problem soils Acid soils Acid sulphate soils Calcareous soils Submerged soils
- 8. Saline Soils The soils have neutral soluble salts hence saturation paste extract pHe < 8.5. These soils have neutral soluble salts of Na, Ca and Mg with chlorides and sulphates hence electrical conductivity of saturation extract ECe is generally more than 4 d Sm-1 at 250C. Dominant salts are NaCl, CaCl, MgCl, or NaSO4, CaSO4, MgSO4. The excess of neutral soluble salts that limits the normal plant growth. Total soluble salts conce. > 0.1% Soils remain flocculated condition pH: Varies 7.5 to 8.5 ECe: > 4 dSm-1 ESP: < 15% Total soluble salt content is > 0.1 % White colour due to NaCl and Na2SO4 Brown colour saline soil is due to NaNO3 Characteristics of saline soils
- 9. Measurement of Salinity Dissolved ions and two metal plates Voltage is applied & ions move toward oppositely charged plates Electrical Conductivity (EC) is an measure of the flow of electricity through a material Saline soils and salty water conduct more electricity than nonsaline soils or pure water. It is the ions that pass or conduct electricity from one ion to the next As salt concentration increases, EC increases. Acidic or low pH solutions also exhibit high EC Expressed in dS/m (SI units) or mmhos/cm (old unit) dS/m = mmhos/cm Use an EC ‘bridge’ or meter to measure how well water extracted from soil can conduct electricity
- 10. Deionized water: 0.0005 to 0.002 dS m-1 Seawater: 40 to 55 dS m-1 Poor quality irrigation water: > 3 dS m-1 Saturated Paste Extract EC of saline soils: ≥ 4 dS m-1 EC values for common waters (dS/m)
- 11. TDS – Total dissolved solids – Cations + anions + anything <2 microns – Good quality water has <500 mg/L or ppm TDS – measure using gravimetry or EC • Evaporate water off and accurately weigh the residue • Problematic due to hydration and volatilization – EC (dS/m) x 640 ≈ TDS (mg/L) • TDS ‘meters’ are really EC meters with conversion factor Measurement of Salinity – TDS
- 12. Charecteristcis of Saline Soils 1 Excess soluble salts like NaCl, CaCl, MgCl, or NaSO4, CaSO4, MgSO4. 2 Correspondence to Hilard’s (1906) white alkali soils due to white layer of soluble salts over the surface 3 In Russian such soils are called “Solonchak,s” 4 Saline soils can be recognized by the presence of white salt crust on the surface 5 If adequate drainage is available, excess soluble salts may be removed by leaching and these soils can be normal 6 Saline soils can be determined by the kind and amount of salts present.
- 13. Features of Saline Soils 7 Amount of soluble salts present controls the osmotic pressure of the soil solution 8 Na seldom comprises more than half of the soluble cations and hence is not adsorbed to any significant extent 9 Chief anions are chlorides, sulphates and sometimes nitrates and some amount of bicarbonate may occur but carbonates are invariably absent. 10 Generally deficient in P, Zn, Fe, N 11 Soils are aggregated and highly permeable 12 Soils are flocculated and hence drainage is good.
- 14. Saline soils
- 18. Alkali soils/sodic soils Non-Saline Alkali Soils pH= 8.5 to 10 ECe = < 4.0 d Sm-1 ESP = >13% TSS = < 1% Saline-Alkali Soils pH= >8.5 ECe = > 4.0 d Sm- 1 ESP = >15% TSS = > 1% Degraded Alkali soils pH= > 8.5 (Surface layer acidic) ECe = < 4.0 d Sm-1 ESP = >15% TSS = > 1% Black colour Prism like structure Compact
- 19. Features of Non-Saline Alkali / Sodic Soils 1 Hilard’s Black Alkali Soil and Russian term Solonetz 2 Occurs in Arid and Semiarid regions in small irregular areas while are often termed as “slick spots” 3 Except when gypsum is present in the soil or irrigation water, the drinage and leaching of saline-alkali soils leads to formation of non-saline alkali soils 4 Removal of excess salts in such soils tends to increase in the rate of hydrolysis of the exchangeable Na which causes rise in pH. Na + H2O= NaOH 5 Dispersed and dissolved OM present in the soil solution of alkaline soils may be deposited on the soil surface by evaporation thus causing darkening and giving rise to the term “Black Alkali”
- 20. Features of Non-Saline Alkali / Sodic Soils 6 If allowed sufficient time, these soils develop characteristic morphological feature…Because partially sodium saturated clay is highly dispersed it may be transported downward through the soil and accumulates at lower level…..this causes….. 7 Few inches of the surface soil may have relatively coarse texture and friable; but below, where the clay accumulates, the soil may develop dense layer of low permeability that have COLUMNAR OR PRISMATIC structure, thereby difficult to till. 8 Exch. Na has marked effect on physical and chemical properties of soil. As the proportion of Na increases- dispersion of soil increases 9 At high pH reading and in the presence of carbonates ions, Ca and Mg are precipitated; hence the soil solution of this non- saline alkali soils usually contain only small amount of theses cations. 10 Deficient in P/Ca/N/Fe/Zn
- 21. 1 ESP= >15% pHe = > 8.5 EC = > 4.0 dS-1 2 Formed due to salinization and alkalinization…. As long as excess salts are present, the apperance and properties of theses soils are generally similar to saline soils 3 If excess soluble salts are leached downward, the properties of these soils then it shows similar features like non-saline alkali soils 4 As the concentration of salts in the soil solution is lowered, some of the Exch.Na hydrolyses and forms NaOH. Na + H2O = NaOH 5 This may change to NaCO3 upon reaction with CO2 absorbed from the atmosphere..NaOH + CO2 = Na2CO3 (Strongly alkaline) 6 Na causes dispersion of clay and soil becomes unfavorable for entry and movement of water and even for tillage Features of Saline Alkali Soils
- 22. Formation of Saline-Alkali Soils-Reasons 1 Arid and semiarid region 2 Poor drainage 3 High water table 4 Overflow of sea water over land 5 Introduction of irrigation water 6 Salts blown by wind 7 Saline nature of parent rock material 8 Excessive use of basic fertilizers 9 Humid and semi humid region
- 23. 1) Arid and Semi-arid region Annual rainfall is less-not sufficient to leach down the salts Evaporation rate is low Intensity of salinization… increases with dryness of climate 2) Poor drainage of soil During high rainfall-salts are leached fro upper layer to lower layer if drainage is impeded, they will accumulate in lower layer When water evaporates again salts move towards upward layer causes secondary salinization
- 24. 3) High water table Groundwater in arid- semiarid region usually contains considerable amount of soluble salts High water table causes water to move upwards towards surface by capillary action causes secondary salinization 4) Overflow of sea water Low laying areas near sea which gets sea water during high tides thereby accumulate the salts
- 25. 5) Introduction of irrigation water Clay soil, impeded drainage, injudicious use of water, monocropping, faulty irrigation methods, excess irrigation Ground water is the arid regions is saline in nature. It has excess soluble salts 6) Salts blown by wind In arid regions near the sea, a lots of salts are blown by wind year after year and gets deposited on surface. Due to low rainfall, they are not washed/leached out back to sea and added in soil.Rajasthan
- 26. Effect of SALINE soil on Soil/Plant/Microbes 1 Neutral soluble salts of chlorides and sulphates are more 2 Excess soluble salts increases OP in soil than cell sap. 3 This prevents the absorption of moisture and nutrients. 4 Excess salts produces toxic effects 5 Specific ion toxicity 6 Germination & root growth affected 7 Secondary salinization affects root growth 8 Less microbial population & activity 9 Less nutrients availability/more dose 10 Deficiency of macro and micronutrients (P, Zn,Fe, Mn) 11 Ca and Mg decreases zeta potential causing reduction in thickness of DDL-enhances flocculation 12 Soils are aggregated & highly permeable, drainage is good
- 29. Effect of SODIC soil on Soil/Plant/Microbes 1 Insoluble salts 2 Excess insoluble salts of Na enhances Na2CO3 enhances alkalinity 3 Na causes dispersion of clay and destroys soil structure 4 Na is monovalent adsorbs on clay and enhances the zeta potential which enhances thickness of DDL 5 Sealing of macro & micro pores that reduces aeration Reduced aeration cusses.. 7 less microbial activity, permeability reduced microbial activity reduced , gas exchange inhibits, compactness increases, impeded drainage, stagnation of water, BD increases 8 Availability of P,Ca, N, Fe, Mn and Zn reduces 9 Germination, root growth, microbial activity & population reduced 10 Root growth, tillering, flowering, fruiting, shelf life of fruits reduced .
- 30. Reclamation of Saline Soils Mechanical Methods Flooding and leaching down of the soluble salts Scrapping of the surface soil Cultural methods 1.Providing proper drainage 2. Use of salt free irrigation water 3. Judicious use of IW 4. Planting/sowing of seed in furrow 5. Use of acidic fertilizers 6. Use of organic manures 7. Ploughing & leveling of land 8. Mulching or retardation of evaporation of water from soil surface 9. Growing of salt tolerant crops
- 31. Mechanical method A combination of flooding after drain is most effective to leach down the soluble salts which are neutral and high in Ca and Mg and very little exch.Na. Scarpping of salts Leaching requirement (LR) Defined as the fraction of irrigation water that must be leached through root zone to control the salinity at any specified levels. LR is a simply ratio of drainage water to the depth of irrigation water. LR = Ddw/Diw X 100 Where LR = Leaching requirement Ddw = Depth of drainage water Diw = Depth of irrigation water LR = ECiw/Ddw X 100 ECiw = EC of irrigation water ECdw = Ec of drainage water
- 32. Example; For irrigation water with Ex 1, 2, 3 dS m-1 the leaching requirement will be 13, 25 and 28% respectively value of EC Taking ECdw is 8 dSm-1 LR= 1/8 X 100 = 12.5%=13% LR = 2/8X 100 = 25 %
- 33. Reclamation of Alkali Soils Chemical Methods 1. Application of Gypsum and gypsum requirement 2.Use of sulphur/Iron pyrite 3. Addition of sulphuric acid 4.Additio of organic matter 5. Addition of molasses Cultural methods/ Management practices 1. Water management and cropping system are very important 2. Growing of salt tolerant crops and crop verities
- 34. Gypsum requirement: Amount of gypsum required to be added to sodic soil to lower the ESP to desired value is called Expressed as meq Ca2+ per 100 g soil According to Schoonover one equivalent of gypsum corresponds to 1.72 t gypsum/acre foot of soil GR depends on Exchangeable Na content to be exchanged Exchange efficiency Depth of soil to be reclaimed GR= ESP1 –ESP2/100 X ECE of soil Where ESP 1= Actual ESP of sodic soil ESP2 = Desired ESP of soil CEC= Cation Exchange capasity
- 35. Soil amendments These are substances that influence the plant growth favorably by producing in the soil one or more of the following beneficial effects. • Changing the reaction ,that is making the soil less acidic or less alkaline ; • Changing the plant nutrients in the soil from unavailable to available forms; • Improving the physical conditions of the soil and • Counteracting the effects of injurious substances Soil amendments usually contain plant nutrients also .Agricultural liming materials, for example, supply calcium and, sometimes magnesium as nutrient el
- 36. Na2CO3 + CaSO4 = CaSO3 + Na2SO4 (leachable) Gypsum Gypsum is chemically CaSO4.2H2O and is a white mineral that occurs extensively in natural deposits. It must be ground before it is applied to the soil. Gypsum is soluble in water to the extent of about one- fourth of 1 percent and is, therefore, a direct source of soluble calcium. Gypsum reacts with both the Na2CO3, and the adsorbed sodium as follows: Calcium chloride Calcium chloride is chemically CaCl2 2H2O. It is a highly soluble salt which supplies soluble calcium directly. Its reactions in sodic soil are similar to those of gypsum: Na2CO3 + CaCl2= CaCO3 + 2 NaCl (leachable)
- 37. Sulphuric acid Sulphuric acid is chemically H2SO4. It is an oily corrosive liquid and is usually about 95 percent pure. Upon application to soils containing calcium carbonate it immediately reacts to form calcium sulphate and thus provides soluble calcium indirectly. Chemical reactions involved are: Na2CO3 + H2SO4 = CO2 + H2O + Na2SO4 (leachable) CaCO3 + H2SO4 = CaSO4 + H2O + CO2
- 40. Iron sulphate and aluminium sulphate (alum) Chemically these compounds are FeSO4.7H2O and Al2(SO4)3.18H2O respectively. Both these solid granular materials usually have a nigh degree of purity and are soluble in water. When applied to soils, these compounds dissolve in soil water and hydrolyse to form sulphuric acid, which in turn supplies soluble calcium through its reaction with lime present in sodic soils. Chemical reactions involved are: FeSO4 + 2H2O = H2SO4 + Fe (OH)2 H2SO4 + CaCO3 = CaSO4 + H2O + CO2 Similar reactions are responsible for the improvement of sodic soils when aluminium sulphate is used as an amendment.
- 41. Sulphur (S) Sulphur is a yellow powder ranging in purity from 50 percent to more than 99 percent. It is not soluble in water and does not supply calcium directly for replacement of adsorbed sodium. When applied for sodic soil reclamation, sulphur has to undergo oxidation to form sulphuric acid which in turn reacts with lime present in the soil to form soluble calcium in the form of calcium sulphate: 2 S + 3 O2 = 2 SO3 (microbiological oxidation) SO3 + H2O = H2SO4 H2SO4 + CaCO3 Û CaSO4 + H2O + CO2
- 42. Pyrite Pyrite (FeS2) is another material that has been suggested as a possible amendment for sodic soil reclamation. Reactions leading to oxidation of pyrite are complex and appear to consist of chemical as well as biological processes. The following sequence has been proposed for the oxidation of pyrite by Temple and Delchamps (1953). The first step in the oxidation is non biological and iron II sulphate (ferrous) is formed
- 43. 2 FeS2 + 2 H2O + 7 O2 = 2 FeSO4 + 2 H2SO4 This reaction is then followed by the bacterial oxidation of iron II sulphate, a reaction normally carried out by Thiobacillus ferrooxidans, 4 FeSO4 + O2 +2 H2SO4 = 2 Fe2 (SO4)3 + 2 H2O Subsequently iron III sulphate (ferric) is reduced and pyrite is oxidized by what appears to be a strictly chemical reaction. Fe2 (SO4)3 + FeS2 = 3 FeSO4 +2 S Elemental sulphur so produced may then be oxidized by T. thiooxidans and the acidity generated favours the continuation of the process 2 S + 3 O2 + 2 H2O = 2 H2SO4 Summary: 4 FeS2 + 2 H2O + 15 O2 = 2 Fe2 (SO4)3 + 2 H2SO4
- 44. Salt Tolerant Crops High Medium Low Barely Castor Pea Sesabania Cotton Sunhemp Rice Sorghum Gram Sugarcane Pearl millet Linseed Oats Maize Sesamum Berseem Mustard Lucerne Wheat
- 45. Salt Tolerance on the Basis of EC 1 No effects on crop 2 - 4 dSm-1 2 Sensitive crop – restricted yield 4 - 8 dSm-1 3 Many crops restricted yield 8 - 16 dSm-1 4 Most crops restricted yield > 16 dSm-1