2014 BDSRA Hofmann Enzyme Replacement Therapy (ERT)
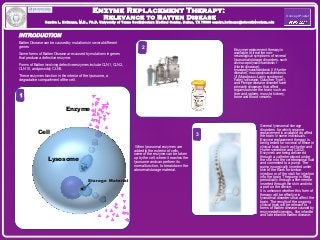
- 1. Enzyme Replacement TThheerraappyy:: RReelleevvaannccee ttoo BBaatttteenn DDiisseeaassee SSaannddrraa LL.. HHooffmmaannnn,, MM..DD..,, PPhh..DD.. UUnniivveerrssiittyy ooff TTeexxaass SSoouutthhwweesstteerrnn MMeeddiiccaall CCeenntteerr,, DDaallllaass,, TTXX 7755339900 ssaannddrraa..hhooffmmaannnn@@uuttssoouutthhwweesstteerrnn..eedduu INTRODUCTION Batten Disease can be caused by mutations in several different genes. Some forms of Batten Disease are caused by mutations in genes that produce a defective enzyme. Forms of Batten involving defective enzymes include CLN1, CLN2, CLN10, and possibly CLN5. These enzymes function in the interior of the lysosome, a degradative compartment of the cell. Enzyme replacement therapy is available to treat the non-neurological symptoms of several lysosomal storage disorders, such as mucopolysaccharidosis I (Hurler disease), mucopolysaccharidosis II (Hunter disease), mucopolysaccharidosis VI (Maroteaux-Lamy syndrome), Fabry’s disease, Gaucher Type I, and Pompe disease disorders are primarily diseases that affect organs outside the brain, such as liver and spleen, muscle, kidney, bone and blood vessels. When lysosomal enzymes are added to the exterior of cells, some of the enzyme can be taken up by the cell, where it reaches the lysosome and can perform its normal function, to break down the abnormal storage material. Concept Poster Enzyme Cell Storage Material Lysosome 1 3 2 Several lysosomal storage disorders for which enzyme replacement is available do affect the brain in some individuals. Enzyme replacement therapy is being tested for several of these in clinical trials (such as Hunter and Hurler syndrome and CLN2). Enzymes are being delivered through a catheter placed under the skin into the cerebrospinal fluid and connected to a pump. The pump is surgically inserted under skin of the flank for lumbar injection or of the skull for injection into the brain. The pump is filled periodically through a fine needle inserted through the skin and into a port on the device. It is unknown whether this form of therapy will be effective in lysosomal disorders that affect the brain. The results of the ongoing clinical trials will be relevant to forms of Batten disease caused by enzyme deficiencies, like infantile and late infantile Batten disease.
