Separation of substances
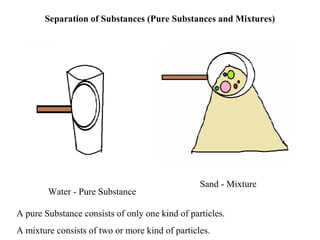
- 1. Separation of Substances (Pure Substances and Mixtures) Sand - Mixture Sand - Mixture Water - Pure Substance A pure Substance consists of only one kind of particles. A mixture consists of two or more kind of particles.
- 2. Types of Mixture A homogeneous mixture is that which has the same composition throughout, that is, its components are uniformly distributed and cannot be distinguished from each other. A heterogeneous mixture is that which does not have the same composition throughout, that is, its components are not uniformly distributed and can be distinguished from each other
- 3. Separating the components of Mixtures a. Mixture of Solids with Solids i. Hand Picking The components of a solid-solid mixture can be separated by hand picking. For e.g. separating pebbles from rice or dal, separating grass from mint leaves.
- 4. ii. Threshing This method is generally used by the farmers to separate the grains from the stalks after harvesting. The dried stalks are beaten or threshed to separate the grains. However, in large farms threshing is done by using threshing machines.
- 5. iii. Winnowing Winnowing In this method, the mixture is allowed to fall from a height. The lighten components get separated from the heavier ones because of wind or air blow. This method is used to separate lighter husk from heavier Grains like wheat.
- 6. iv. Sieving This method is used to separate the components that are of different sizes. For sieving, a sieve is used. Sieve is a wire mesh fixed tightly in a frame.
- 7. v. Magnetic Separation In this magnetic substances (like iron get attracted by the magnet. For e.g.- when a magnet is brought near iron pins, all pins get clinged to it.
- 8. vi. Sublimation The process in which a solid changes directly into gaseous state on heating is called sublimation.
- 9. b. Mixture of solids with liquids i. Evaporation Evaporation is a process in which a liquid changes into gaseous form on heating is called evaporation.
- 10. ii. Crystallization Crystallization is a process which separates a pure solid in the form of its crystals from a saturated solution.
- 11. 2. Separating solids that do not dissolve in liquids i. Sedimentation and Decantation Sedimentation is the process by which the insoluble, heavy solid particles settle down their own in a solution. In order to separate the two, the liquid has to be gently poured into another container without disturbing the sediments. This process of obtaining clear liquid by pouring it into another container without disturbing the sediments is called decantation.
- 12. ii. Loading Loading is a process which speeds up the sedimentation. In fact, loading is a faster process as compared to sedimentation.
- 13. iii. Filtration Filtration is a process of separating insoluble solids (like mud, tea leaves etc.) from a liquid using fine pores of the filter.
- 14. Separating immiscible liquids It is a mixture of two liquids which do not mix with each other.
- 15. MADE BY: RITISHA SINGH CLASS: 6 C th ROLL NO: 27
