N butane and cyclohexane
•Download as DOCX, PDF•
0 likes•780 views
assignment of n-butane and cyclohexane.
Report
Share
Report
Share
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
METHODS OF DETERMINATION OF CONFIGURATION OF GEOMETRICAL ISOMERS.pptx

METHODS OF DETERMINATION OF CONFIGURATION OF GEOMETRICAL ISOMERS.pptx
Practical Experiment 6: To synthesize and characterized 2,3 diphenyl quninox...

Practical Experiment 6: To synthesize and characterized 2,3 diphenyl quninox...
Synthesis and reactions of Seven membered heterocycle-Azepines

Synthesis and reactions of Seven membered heterocycle-Azepines
Unit V: Reaction of synthetic importance as per PCI Syllabus of POC-III

Unit V: Reaction of synthetic importance as per PCI Syllabus of POC-III
Oxazole - Synthesis of Oxazole - Reactions of Oxazole - Medicinal uses of Oxa...

Oxazole - Synthesis of Oxazole - Reactions of Oxazole - Medicinal uses of Oxa...
Pyrazole - Synthesis of Pyrazole - Characteristic Reactions of Pyrazole - Med...

Pyrazole - Synthesis of Pyrazole - Characteristic Reactions of Pyrazole - Med...
STEREOSPECIFIC REACTION, STEREOSELECTIVE REACTION, OPTICAL PURITY, ENANTIOMER...

STEREOSPECIFIC REACTION, STEREOSELECTIVE REACTION, OPTICAL PURITY, ENANTIOMER...
Similar to N butane and cyclohexane
Similar to N butane and cyclohexane (20)
Bayers theory & conformational analysis of cylohexane

Bayers theory & conformational analysis of cylohexane
Conformational analysis of ethane butane aliphatics

Conformational analysis of ethane butane aliphatics
Chapter 04 stereochemistry of alkanes and cycloalkanes

Chapter 04 stereochemistry of alkanes and cycloalkanes
More from Sameen Fatima
More from Sameen Fatima (20)
Recently uploaded
https://app.box.com/s/7hlvjxjalkrik7fb082xx3jk7xd7liz3TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...Nguyen Thanh Tu Collection
Making communications land - Are they received and understood as intended? webinar
Thursday 2 May 2024
A joint webinar created by the APM Enabling Change and APM People Interest Networks, this is the third of our three part series on Making Communications Land.
presented by
Ian Cribbes, Director, IMC&T Ltd
@cribbesheet
The link to the write up page and resources of this webinar:
https://www.apm.org.uk/news/making-communications-land-are-they-received-and-understood-as-intended-webinar/
Content description:
How do we ensure that what we have communicated was received and understood as we intended and how do we course correct if it has not.Making communications land - Are they received and understood as intended? we...

Making communications land - Are they received and understood as intended? we...Association for Project Management
Recently uploaded (20)
Food safety_Challenges food safety laboratories_.pdf

Food safety_Challenges food safety laboratories_.pdf
Python Notes for mca i year students osmania university.docx

Python Notes for mca i year students osmania university.docx
On National Teacher Day, meet the 2024-25 Kenan Fellows

On National Teacher Day, meet the 2024-25 Kenan Fellows
General Principles of Intellectual Property: Concepts of Intellectual Proper...

General Principles of Intellectual Property: Concepts of Intellectual Proper...
UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf

UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf
HMCS Vancouver Pre-Deployment Brief - May 2024 (Web Version).pptx

HMCS Vancouver Pre-Deployment Brief - May 2024 (Web Version).pptx
Sensory_Experience_and_Emotional_Resonance_in_Gabriel_Okaras_The_Piano_and_Th...

Sensory_Experience_and_Emotional_Resonance_in_Gabriel_Okaras_The_Piano_and_Th...
TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...
Making communications land - Are they received and understood as intended? we...

Making communications land - Are they received and understood as intended? we...
N butane and cyclohexane
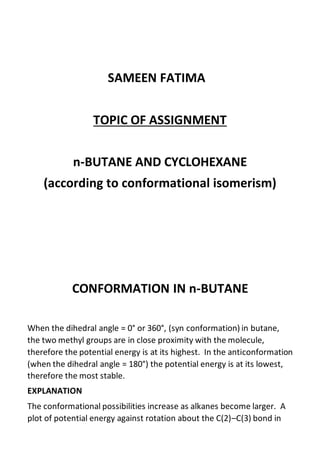
- 1. SAMEEN FATIMA TOPIC OF ASSIGNMENT n-BUTANE AND CYCLOHEXANE (according to conformational isomerism) CONFORMATION IN n-BUTANE When the dihedral angle = 0° or 360°, (syn conformation) in butane, the two methyl groups are in close proximity with the molecule, therefore the potential energy is at its highest. In the anticonformation (when the dihedral angle = 180°) the potential energy is at its lowest, therefore the most stable. EXPLANATION The conformational possibilities increase as alkanes become larger. A plot of potential energy against rotation about the C(2)–C(3) bond in
- 2. butane is shown below. The lowest-energy arrangement, called the antiperiplanar (or anti) conformation, is the one in which the two large methyl groups are as far apart as possible. As rotation around the C(2)–C(3) bond occurs, another eclipsed conformation (anticlinal) is reached in which there are two Me–H interactions and one H–H interaction. If we assign the energy value (4 kJ/mol) for H–H eclipsing interactions that was previously derived from ethane, we can predict that each Me–H interaction in the anticlinal conformation costs about 5 kJ/mol. As bond rotation continues, an energy minimum is reached at the staggered conformation where the methyl groups are 60° apart (a gauche relationship). This lies 3.2 kJ/mol higher in energy than the anti conformation even though it has no eclipsing interactions. This energy difference is due to the fact that the hydrogen atoms of the methyl groups are near each other in the gauche conformation, resulting in steric strain, which is the repulsive interaction that occurs when atoms would otherwise tend to occupy the same space. As the dihedral angle between the methyl groups approaches 0°, an energy maximum is reached. The methyl groups are forced even closer together than in the gauche conformation, and both torsional strain
- 3. and steric strain are present. A total strain energy of 25 kJ/mol has been estimated for this conformation, allowing us to calculate a value of 17 kJ/mol for the Me–Me eclipsing interaction. Completing the 360° rotation after the synperiplanar point produces the mirror images of what we've already seen; another gauche conformation, another eclipsed conformation and finally a return to the anti conformation. Use the interactive model below to explore the conformations of butane. In particular, try the following 'visualisation' of steric strain: Select Reset, Atoms 100%, coloured methyls and Animate loop, then watch the interactions of the two methyl groups as they pass by each other. The butane structure used in this animation was generated by quantum chemical calculation using the 6-31G* basis set. Eclipsed conformations Totally eclipsed partially eclipsed partially eclipsed 0o (I) 120O (II) 240O (III) Staggered conformations IV V VI 60O (gouche) 180o (anti) 300o (gouche) CONFORMATION IN CYCLOHEXANE
- 4. A cyclohexane conformation is any of several three-dimensional shapes adopted by a cyclohexane molecule. Because many compounds feature structurally similar six-membered rings, the structure and dynamics of cyclohexane are important prototypes of a wide range of compounds.[1][2] PRINCIPLE CONFORMERS Chair conformation The chair conformation is the most stable conformer. At 25 °C, 99.99% of all molecules in a cyclohexane solution adopt this conformation. The symmetry is D3d. All carbon centers are equivalent. Six hydrogen centers are poised in axial positions, roughly parallel with the C3 axis. Six hydrogen atoms are poised nearly perpendicular to the C3 symmetry axis. These H atoms are respectively referred to as axial and equatorial. Each carbon bears one "up" and one "down" hydrogen. The C-H bonds in successive carbons are thus staggered so that there is little torsional strain. The chair geometry is often preserved when the hydrogen atoms are replaced by halogens or other simple groups. Boat conformation The boat conformations have higher energy than the chair conformations. The interaction between the two flagpole hydrogens, in particular, generates steric strain. Torsional strain also exists between the C2–C3 and C5–C6 bonds, which are eclipsed. Because of this strain, the boat configuration is unstable (i.e. is not a local energy minimum). The symmetry is C2v.
- 5. The boat conformations spontaneously distorts to twist-boat conformations. Here the symmetry is D2, a purely rotational point group. This conformation can be derived from the boat conformation by applying a slight twist to the molecule so as to remove eclipsing of two pairs of methylene groups. The concentration of the twist-boat conformation at room temperature is less than 0.1%, but at 1073 kelvins it can reach 30%. Rapid cooling of a sample of cyclohexane from 1073 K to 40 K will freeze in a large concentration of twist-boat conformation, which will then slowly convert to the chair conformation upon heating. Boat conformation AXIAL AND EQUILIBRIUM BONDS IN CYCLOHEXANE The bonds in cyclohexane conformation are shown below in structures. TWISTED BOAT
