2011 topic 09 voltaic cells sl
•Download as PPTX, PDF•
3 likes•3,023 views
Report
Share
Report
Share
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
CBSE Class 12 Chemistry Chapter 10 (Haloalkanes and Haloarenes) | Homi Institute

CBSE Class 12 Chemistry Chapter 10 (Haloalkanes and Haloarenes) | Homi Institute
Viewers also liked
Viewers also liked (15)
IB Chemistry on Voltaic Cell, Standard Electrode Potential and Standard Hydro...

IB Chemistry on Voltaic Cell, Standard Electrode Potential and Standard Hydro...
Similar to 2011 topic 09 voltaic cells sl
Similar to 2011 topic 09 voltaic cells sl (20)
Ion selective electrodes ISE (potentiometery) PH meter

Ion selective electrodes ISE (potentiometery) PH meter
More from David Young
More from David Young (20)
2016 topic 0 - oxidation and reduction (INTRODUCTION)

2016 topic 0 - oxidation and reduction (INTRODUCTION)
Recently uploaded
Recently uploaded (20)
Web Form Automation for Bonterra Impact Management (fka Social Solutions Apri...

Web Form Automation for Bonterra Impact Management (fka Social Solutions Apri...
Exploring the Future Potential of AI-Enabled Smartphone Processors

Exploring the Future Potential of AI-Enabled Smartphone Processors
Strategies for Landing an Oracle DBA Job as a Fresher

Strategies for Landing an Oracle DBA Job as a Fresher
Emergent Methods: Multi-lingual narrative tracking in the news - real-time ex...

Emergent Methods: Multi-lingual narrative tracking in the news - real-time ex...
Apidays New York 2024 - The value of a flexible API Management solution for O...

Apidays New York 2024 - The value of a flexible API Management solution for O...
ProductAnonymous-April2024-WinProductDiscovery-MelissaKlemke

ProductAnonymous-April2024-WinProductDiscovery-MelissaKlemke
Apidays New York 2024 - Passkeys: Developing APIs to enable passwordless auth...

Apidays New York 2024 - Passkeys: Developing APIs to enable passwordless auth...
Finding Java's Hidden Performance Traps @ DevoxxUK 2024

Finding Java's Hidden Performance Traps @ DevoxxUK 2024
Why Teams call analytics are critical to your entire business

Why Teams call analytics are critical to your entire business
CNIC Information System with Pakdata Cf In Pakistan

CNIC Information System with Pakdata Cf In Pakistan
How to Troubleshoot Apps for the Modern Connected Worker

How to Troubleshoot Apps for the Modern Connected Worker
Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood

Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood
Spring Boot vs Quarkus the ultimate battle - DevoxxUK

Spring Boot vs Quarkus the ultimate battle - DevoxxUK
"I see eyes in my soup": How Delivery Hero implemented the safety system for ...

"I see eyes in my soup": How Delivery Hero implemented the safety system for ...
2011 topic 09 voltaic cells sl
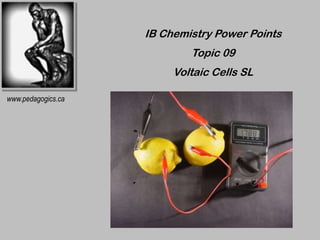
- 1. IB Chemistry Power Points Topic 09 Voltaic Cells SL www.pedagogics.ca
- 2. ELECTROCHEMISTRY Deals with chemical changes produced by an electric current and with the production of electricity by chemical reactions All electrochemical reactions involve transfer of electrons and are redox reactions Electrochemical reactions take place in electrochemical cell (an apparatus that allows a reaction to occur through an external conductor)
- 3. ELECTROCHEMICAL CELLS Two types: Voltaic cells: produce electrical energy Also called galvanic cells. In these cells spontaneous chemical reactions generate electrical energy and supply it to an external circuit. Electrolytic cells: require electrical energy These are cells in which an external electrical source forces a nonspontaneous reaction to occur.
- 4. Electrochemical Terms – all cells Electrode: A conductor used to establish contact with a nonmetallic part of a circuit, such as an electrolyte Half-cell: a metal electrode in contact with a solution of its own ions Anode: The electrode where oxidation takes place Cathode: The electrode where reduction takes place
- 5. VOLTAIC CELLS • Cells in which spontaneous reactions produces electrical energy • The two half-cells are separated so that electron transfer occurs through an external circuit • Each half-cell contains the oxidized and reduced forms of a species in contact with each other • Half-cells are linked by a piece of wire and a salt bridge
- 6. A TYPICAL VOLTAIC CELL
- 7. The Zinc-Copper cell Composed of two half-cells: 1. A strip of copper immersed in 1 M CuSO4 2. A strip of zinc immersed in 1 M ZnSO4 Experimentally we see: Initial voltage is 1.10 volts The mass of the zinc electrode decreases The mass of the copper electrode increases [Zn2+] increases and [Cu2+] decreases
- 9. Zinc – Copper Cell - notation Salt bridge Zn Zn 2+ (1.0 M) Cu 2+ (1.0 M) Cu Electrode Species (with concentrations) in contact with electrodes
- 10. A salt bridge (or porous partition) has three functions: 1. The salt bridge allows for the flow of ions and therefore electrical contact between the two half-cells 2. As a result of electrical contact, the salt bridge maintains the electrical neutrality in each half-cell as ions flow into and out of the salt bridge 3. The salt bridge prevents mixing of the electrode solutions
- 11. Electrical neutrality in each half cell is important! anions flow into the oxidation half-cell to counter the build-up of positive charge Current flows spontaneously from and vice versa negative to the positive electrode (oxidation electrode to reduction If this did not electrode) happen, current would stop flowing In voltaic cells, voltage drops as the reaction proceeds. When voltage = 0, the reaction is at equilibrium
- 12. Voltaic Cells - Summary Cathode: Anode: reduction oxidation positive negative Voltaic cells: Electrochemical cells in which a spontaneous redox reaction can be harnessed to produce an electric current.
- 13. Example: The Zinc – Copper Cell
