Quantum physics
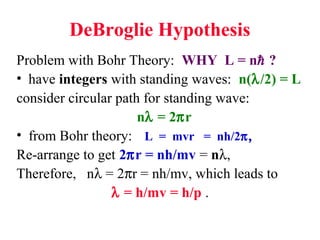
- 1. DeBroglie Hypothesis Problem with Bohr Theory: WHY L = n ? • have integers with standing waves: n(λ/2) = L consider circular path for standing wave: nλ = 2πr • from Bohr theory: L = mvr = nh/2π, Re-arrange to get 2πr = nh/mv = nλ, Therefore, nλ = 2πr = nh/mv, which leads to λ = h/mv = h/p .
- 2. DeBroglie Hypothesis λDeBroglie = h/mv = h/p In this case, we are considering the electron to be a WAVE, and the electron wave will “fit” around the orbit if the momentum (and energy) is just right (as in the above relation). But this will happen only for specific cases - and those are the specific allowed orbits (rn) and energies (En) that are allowed in the Bohr Theory!
- 3. DeBroglie Hypothesis The Introduction to Computer Homework on the Hydrogen Atom (Vol. 5, number 5) shows this electron wave fitting around the orbit for n=1 and n=2. What we now have is a wave/particle duality for light (E&M vs photon), AND a wave/particle duality for electrons!
- 4. DeBroglie Hypothesis If the electron behaves as a wave, with λ = h/mv, then we should be able to test this wave behavior via interference and diffraction. In fact, experiments show that electrons DO EXHIBIT INTERFERENCE when they go through multiple slits, just as the DeBroglie Hypothesis indicates.
- 5. DeBroglie Hypothesis Even neutrons have shown interference phenomena when they are diffracted from a crystal structure according to the DeBroglie Hypothesis: λ = h/p . Note that h is very small, so that normally λ will also be very small (unless the mv is also very small). A small λ means very little diffraction effects [1.22 λ = D sin(θ)].
- 6. Quantum Theory What we are now dealing with is the Quantum Theory: • atoms are quantized (you can have 2 or 3, but not 2.5 atoms) • light is quantized (you can have 2 or 3 photons, but not 2.5) • in addition, we have quantum numbers (L = n , where n is an integer)
- 7. Heisenberg Uncertainty Principle There is a major problem with the wave/particle duality: a) a wave with a definite frequency and wavelength (e.g., a nice sine wave) does not have a definite location. [At a definite location at a specific time the wave would have a definite phase, but the wave would not be said to be located there.] [ a nice travelling sine wave = A sin(kx-ωt) ]
- 8. Heisenberg Uncertainty Principle b) A particle does have a definite location at a specific time, but it does not have a frequency or wavelength. c) Inbetween case: a group of sine waves can add together (via Fourier analysis) to give a semi-definite location: a result of Fourier analysis is this: the more the group shows up as a spike, the more waves it takes to make the group.
- 9. Heisenberg Uncertainty Principle A rough drawing of a sample inbetween case, where the wave is somewhat localized, and made up of several frequencies.
- 10. Heisenberg Uncertainty Principle A formal statement of this (from Fourier analysis) is: ∆x * ∆k = 1/2 (where k = 2π/λ, and ∆ indicates the uncertainty in the value). But from the DeBroglie Hypothesis, λ = h/p, this uncertainty relation becomes: ∆x * ∆(2π/λ) = ∆x * ∆(2πp/h) = 1/2 , or ∆x * ∆p = /2.
- 11. Heisenberg Uncertainty Principle ∆x * ∆p = /2 The above is the BEST we can do, since there is always some experimental uncertainty. Thus the Heisenberg Uncertainty Principle says: ∆x * ∆p > /2 .
- 12. Heisenberg Uncertainty Principle A similar relation from Fourier analysis for time and frequency: ∆t * ∆ω = 1/2 leads to another part of the Uncertainty Principle (using E = hf): ∆t * ∆E > /2 . There is a third part: ∆θ * ∆L > /2 (where L is the angular momentum value). All of this is a direct result of the wave/particle duality of light and matter.
- 13. Heisenberg Uncertainty Principle Let’s look at how this works in practice. Consider trying to locate an electron somewhere in space. You might try to “see” the electron by hitting it with a photon. The next slide will show an idealized diagram, that is, it will show a diagram assuming a definite position for the electron.
- 14. Heisenberg Uncertainty Principle We fire an incoming photon at the electron, have the photon hit and bounce, then trace the path of the outgoing photon back to see where the electron was. electron incoming photon
- 15. Heisenberg Uncertainty Principle electron outgoing photon slit so we can determine direction of the outgoing photon screen
- 16. Heisenberg Uncertainty Principle • Here the wave-particle duality creates a problem in determining where the electron was. electron slit so we can determine direction of the outgoing photon photon hits here
- 17. Heisenberg Uncertainty Principle • If we make the slit narrower to better determine the direction of the photon (and hence the location of the electron, the wave nature of light will cause the light to be diffracted. This diffraction pattern will cause some uncertainty in where the photon actually came from, and hence some uncertainty in where the electron was .
- 18. Heisenberg Uncertainty Principle We can reduce the diffraction angle if we reduce the wavelength (and hence increase the frequency and the energy of the photon). But if we do increase the energy of the photon, the photon will hit the electron harder and make it move more from its location, which will increase the uncertainty in the momentum of the electron.
- 19. Heisenberg Uncertainty Principle Thus, we can decrease the ∆x of the electron only at the expense of increasing the uncertainty in ∆p of the electron.
- 20. Heisenberg Uncertainty Principle Let’s consider a second example: trying to locate an electron’s y position by making it go through a narrow slit: only electrons that make it through the narrow slit will have the y value determined within the uncertainty of the slit width.
- 21. Heisenberg Uncertainty Principle But the more we narrow the slit (decrease ∆y), the more the diffraction effects (wave aspect), and the more we are uncertain of the y motion (increase ∆py) of the electron.
- 22. Heisenberg Uncertainty Principle Let’s take a look at how much uncertainty there is: ∆x * ∆p > /2 . Note that /2 is a very small number (5.3 x 10-35 J-sec).
- 23. Heisenberg Uncertainty Principle If we were to apply this to a steel ball of mass .002 kg +/- .00002 kg, rolling at a speed of 2 m/s +/- .02 m/s, the uncertainty in momentum would be 4 x 10-7 kg*m/s . From the H.U.P, then, the best we could be sure of the position of the steel ball would be: ∆x = 5.3 x 10-35 J*s / 4 x 10-7 kg*m/s = 1.3 x 10-28 m !
- 24. Heisenberg Uncertainty Principle As we have just demonstrated, the H.U.P. comes into play only when we are dealing with very small particles (like individual electrons or photons), not when we are dealing with normal size objects!
- 25. Heisenberg Uncertainty Principle If we apply this principle to the electron going around the atom, then we know the electron is somewhere near the atom, (∆x = 2r = 1 x 10-10 m) then there should be at least some uncertainty in the momentum of the atom: ∆px > 5 x 10-35 J*s / 1 x 10-10 m = 5 x 10-25 m/s
- 26. Heisenberg Uncertainty Principle Solving for p = mv from the Bohr theory [KE + PE = Etotal, (1/2)mv2 - ke2 /r = -13.6 eV gives v = 2.2 x 106 m/s ] gives p = (9.1 x 10-31 kg) * (2.2 x 106 m/s) = 2 x 10-24 kg*m/s; this means px is between -2 x 10-24 kg*m/s and 2 x 10-24 kg*m/s, with the minimum ∆px being 5 x 10-25 kg*m/s, or 25% of p.
- 27. Heisenberg Uncertainty Principle Thus the H.U.P. says that we cannot really know exactly where and how fast the electron is going around the atom at any particular time. This is consistent with the idea that the electron is actually a wave as it moves around the electron.
- 28. Quantum Theory But if an electron acts as a wave when it is moving, WHAT IS WAVING? When light acts as a wave when it is moving, we have identified the ELECTROMAGNETIC FIELD as waving. But try to recall: what is the electric field? Can we directly measure it?
- 29. Quantum Theory Recall that by definition, E = F/q. We can only determine that a field exists by measuring an electric force! We have become so used to working with the electric and magnetic fields, that we tend to take their existence for granted. They certainly are a useful construct even if they don’t exist.
- 30. Quantum Theory We have four LAWS governing the electric and magnetic fields: MAXWELL’S EQUATIONS. By combining these laws we can get a WAVE EQUATION for E&M fields, and from this wave equation we can get the speed of the E&M wave and even more (such as reflection coefficients, etc.).
- 31. Quantum Theory But what do we do for electron waves? What laws or new law can we find that will work to give us the wealth of predictive power that MAXWELL’S EQUATIONS have given us?
- 32. Quantum Theory The way you get laws is try to explain something you already know about, and then see if you can generalize. A successful law will explain what you already know about, and predict things to look for that you may not know about. This is where the validity (or at least usefulness) of the law can be confirmed.
- 33. Quantum Theory Schrodinger started with the idea of Conservation of Energy: KE + PE = Etotal . He noted that • KE = (1/2)mv2 = p2 /2m, and that λ=h/p, so that p = h/λ = (h/2π)*(2π/λ) = k = p, so KE = 2 k2 /2m • Etotal = hf = (h/2π)*(2πf) = ω.
- 34. Quantum Theory He then took a nice sine wave, and called whatever was waving, Ψ: Ψ(x,t) = A sin(kx-ωt) = Aei(kx-ωt ) . He noted that both k and ω were in the exponent, and could be gotten down by differentiating. So he tried operators:
- 35. Quantum Theory Ψ(x,t) = A sin(kx-ωt) = Aei(kx-ωt ) . popΨ = i[dΨ/dx] = i[-ikAe-i(kx-ωt) ] = kΨ = (h/2π)*(2π/λ)*Ψ = (h/λ)Ψ = pΨ . similary: EopΨ = i[dΨ/dt] = i[-iωAei(kx-ωt) ] = ωΨ = ((h/2π)*(2πf)*Ψ = (hf)Ψ = EΨ .
- 36. Quantum Theory Conservation of Energy: KE + PE = Etotal becomes with the momentum and energy operators: -(2 /2m)*(d2 Ψ/dx2 ) + PE*Ψ = i(dΨ/dt) which is called SCHRODINGER’S EQUATION. If it works for more than the free electron, then we can call it a LAW.
- 37. Quantum Theory What is waving here? Ψ What do we call Ψ? the wavefunction Schrodinger’s Equation allows us to solve for the wavefunction. The operators then allow us to find out information about the electron, such as its energy and its momentum.
- 38. Quantum Theory To get a better handle on Ψ, let’s consider light: how did the E&M wave relate to the photon?
- 39. Quantum Theory The photon was the basic unit of energy for the light. The energy in the wave depended on the field strength squared. [Recall energy in capacitor, Energy = (1/2)CV2 , where for parallel plates, Efield = V/d and C// = KεoA/d, so that Energy = (1/2)*(KεoA/d)*(Efieldd)2 = KεoEfield 2 * Vol, or Energyα Efield 2 .]
- 40. Quantum Theory Since Energy is proportional to field strength squared, AND energy is proportional to the number of photons, THEN that implies that the number of photons is proportional to the square of the field strength. This then can be interpreted to mean that the square of the field strength is related to the probability of finding a photon.
- 41. Quantum Theory In the same way, the square of the wavefunction is related to the probability of find the electron! Since the wavefunction is a function of both x and t, then the probability of finding the electron is also a function of x and t! Prob(x,t) = Ψ(x,t)2
- 42. Quantum Theory Different situations for the electron, like being in the hydrogen atom, will show up in Schrodinger’s Equation in the PE part. Different PE functions (like PE = -ke2 /r for the hydrogen atom) will cause the solution to Schrodinger’s equation to be different, just like different PE functions in the normal Conservation of Energy will cause different speeds to result for the particles.
- 43. Schrodinger’s Equation Let’s look at the case of an electron confined to a length, L, but otherwise free. We will first consider the 1-D case. Since the electron is free, PE=0. However, since it is confined to the length, L, we have boundary conditions: Ψ(x=0,t) = 0, and Ψ(x=L,t) = 0. ( / )( / ) ( / )− + = 2 2 2 2 0m x i t∂ ∂ ∂ ∂Ψ Ψ
- 44. Schrodinger’s Equation To solve this differential equation, we use the technique of separation of variables: Ψ(x,t) = X(x)*T(t) . The partial derivative with respect to x does not affect T(t), and the partial derivative with respect to t does not affect X(x). We can also divide both sides of the equation by X(x)*T(t) to get:
- 45. Schrodinger’s Equation Note that the left side depends solely on x, and the right side solely on t. This means that as far as x is concerned, the right side is a constant, and as far as t is concerned, the left side is a constant (which turns out to be the Energy). ( / )( / ) / ( / ) /− = = 2 2 2 2m X x X E i T t T∂ ∂ ∂ ∂
- 46. Schrodinger’s Equation We now have two ordinary differential equations instead of one partial differential equation: -(2 /2m)d2 X/dx2 = E*X for X(x) i dT/dt = E*T for T(t). In fact, any situation where the PE does not explicitly depend on time will have this solution.
- 47. Schrodinger’s Equation i dT/dt = E*T for T(t) The above 1st order ordinary differential equation is easily solved if we recall that the differential of an exponential gives an exponential back again. So try T(t) = Aeat : i(aAeat ) = E(Aeat ) or ia = E, or a = E/i. But we know that E = ω, so this means that: a = ω/i = -iω, and T(t) = A e-iωt .
- 48. Schrodinger’s Equation • Note that the probability depends on Ψ2 (actually, since Ψ can be a complex quantity, Prob(x,t) = Ψ∗ ∗Ψ, where Ψ∗ is the complex conjugate of Ψ). • Note also that T* *T = Ae-iωt * Ae+iωt = A2 , but A can be incorporated as a constant into X(x). Thus the T part of Ψ can effectively be ignored if the PE does not depend on t.
- 49. Schrodinger’s Equation This means that when the PE does not depend on time, Schrodinger’s Equation can be re- written as (since idΨ/dt = E*Ψ) : (-2 /2m)*d2 Ψ/dx2 + PE*Ψ = E*Ψ . Note that Ψ can be complex (just like E could be written in complex form), but the Probability must be real (since it is a measurable quantity). Now we go on to the X equation.
- 50. Schrodinger’s Equation -(2 /2m)d2 X/dx2 = E*X for X(x) This equation can again be solved by inspection since we know that the second derivative of a sine (or cosine) function gives itself back again with a minus sign, so we’ll try X(x) = B sin(kx+φo) : -(2 /2m)(-Bk2 sin(kx+φo)) = E B sin(kx+φo) , or 2 k2 /2m = E (since p = k, E = p2 /2m = KE but this is correct since PE = 0 in this case!)
- 51. Schrodinger’s Equation X(x) = B sin(kx +φo) We now need to apply the Boundary conditions: X(x=0) = 0 works fine for the sine function, but not the cosine function so φo= 0. X(x=L) = 0 demands that kL = nπ, or that k = nπ/L (note that k is quantized, so p is quantized, and E is quantized!).
- 52. Schrodinger’s Equation Note that Schrodinger’s Equation GIVES US A QUANTUM NUMBER. It comes when we apply the boundary conditions! We did not have to put the quantum number into the theory as Bohr did. Note that the Schrodinger’s equation we have written down is for 1-D only (x). We now need to see about a 3-D form.
- 53. Schrodinger’s Equation Momentum is a vector, so the DeBroglie relation: p = k is really a component relation: px = kx, py = ky, pz = kz , where the kx simply describes how the E&M field varies in the x-direction. Thus the pop = i d/dx must now become p i x p i y p i z x op x op z op − − − = ; = = ∂ ∂ ∂ ∂ ∂ ∂ / / ; /
- 54. Schrodinger’s Equation KE = p2 /2m = (px 2 + py 2 + pz 2 )/2m. This can be used in the Schrodinger’s equation with the component operators for p, and using the symbol ∇2 2 2 2 2 2 2 = + +∂ ∂ ∂ ∂ ∂ ∂/ / /x y z
- 55. Schrodinger’s Equation The three dimensional Scrodinger’s Equation becomes (for PE not dependent on time): ( / )− ∇i m2 2 2 + PE = EΨ Ψ Ψ
- 56. Schrodinger’s Equation This equation can also usually be solved by separation of variables, and when applying the boundary conditions (for x, y and z) we usually get THREE QUANTUM NUMBERS (just like we got 1 quantum number in the 1-D case). These quantum numbers come out of the theory rather than being put into the theory as Bohr did in his.
- 57. Quantum Theory From Chemistry, you may recall that FOUR quantum numbers were required, instead of the three that the Schrodinger’s Equation predicts. The fourth quantum number (spin) can be predicted from a relativistic quantum theory, where the equation is called the DIRAC EQUATION. (This also predicts anti-matter as well!)
- 58. Quantum Theory In the hydrogen atom we do not use rectangular coordinates (x,y,z); instead we use spherical coordinates (r,θ,ϕ) because of the spherical symmetry of the potential energy (PE = -ke2 /r). The solution of the Schrodinger’s equation gives us three quantum numbers: n, l, and ml . The fourth number is due to spin, ms .
- 59. Quantum Theory The n quantum number is related to the r equation and is related to energy. The l quantum number is related to the θ equation and is related to angular momentum. The m quantum number is related to the ϕ equation and is related to the z-component of angular momentum, which is related to the magnetic properties of the state.
- 60. Quantum Theory The fourth quantum number, ms, does not have a classical explanation - it is a relativistic quantum phenomenon. It is related to magnetic behavior, and hence has the m name. The closest thing classically we can relate it to is to the case of the electron “spinning”, so that its spinning charge creates a magnetic field. But this does not work out according to classical calculations.
- 61. Pauli Exclusion Principle How do we extend the quantum theory to systems beyond the hydrogen atom? For systems of 2 electrons, we simply have a Ψ that depends on time, and the coordinates of each of the two electrons: Ψ(x1,y1,z1,x2,y2,z2,t) and the Schrodinger’s equation has two kinetic energies instead of one.
- 62. Pauli Exclusion Principle It turns out that the Schodinger’s Equation can again be separated: Ψ = Xa(x1,y1,z1) * Xb(x2,y2,z2) * T(t) . This is like having electron one in state a, and having electron two in state b.
- 63. Pauli Exclusion Principle However, from the Heisenberg Uncertainty Principle (from wave/particle duality), we are not really sure which electron is electron number 1 and which is number 2. This means that the wavefunction must also reflect this uncertainty.
- 64. Pauli Exclusion Principle There are two ways of making the wavefunction reflect the indistinguishability of the two electrons: Ψsym = [Xa(r1)*Xb(r2) + Xb(r1)*Xa(r2) ]* T(t) and Ψanti = [Xa(r1)*Xb(r2) - Xb(r1)*Xa(r2) ]* T(t) .
- 65. Pauli Exclusion Principle Ψsym = [Xa(r1)*Xb(r2) + Xb(r1)*Xa(r2) ]* T(t) Ψanti = [Xa(r1)*Xb(r2) - Xb(r1)*Xa(r2) ]* T(t) . Which (if either) possibility agrees with experiment? It turns out that some particles are explained nicely by the symmetric, and some are explained by the antisymmetric.
- 66. BOSONS Ψsym = [Xa(r1)*Xb(r2) + Xb(r1)*Xa(r2) ]* T(t) Those particles that work with the symmetric form are called BOSONS. All of these particles have integer spin as well. Note that if electron 1 and electron 2 both have the same state, Ψ > 0. This means that both particles CAN be in the same state at the same location at the same time.
- 67. FERMIONS Ψanti = [Xa(r1)*Xb(r2) - Xb(r1)*Xa(r2) ]* T(t) Those particles that work with the anti-symmetric wavefunction are called FERMIONS. All of these particles have half-integer spin. Note that if electron 1 and electron 2 both have the same state, Ψ = 0. This means that both particles CAN NOT be in the same state at the same location at the same time.
- 68. Pauli Exclusion Principle BOSONS. Photons and alpha particles (2 neutrons + 2 protons) are bosons. These particles can be in the same location with the same energy state at the same time. This occcurs in a laser beam, where all the photons are at the same energy (monochromatic).
- 69. Pauli Exclusion Principle FERMIONS. Electrons, protons and neutrons are fermions. These particles can NOT be in the same location with the same energy state at the same time. This means that two electrons going around the same nucleus CAN NOT both be in the exact same state at the same time! This is known as the Pauli Exclusion Principle!
- 70. Pauli Exclusion Principle Since no two electrons can be in the same energy state in the same atom at the same time, chemistry is possible (and so is biology, psychology, sociology, politics, and religion)! Thus, the possibility of chemistry is explained by the wave/particle duality of light and matter, and electrons acting as fermions!
