Lecture7: 123.312
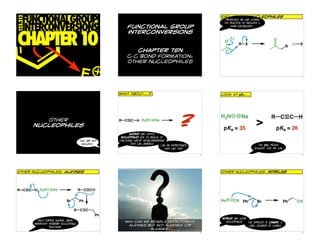
- 1. FUNCTIONALGROUP 123.312 enolates as nucleophiles previously, we had looked INTERCONVERSIONS the reaction of enolates & functional group their equivalents... CHAPTER 10 interconversions Li O O R X Li X R CHAPTER ten c–c bond formation: other nucleophiles E 1 2 3 what about...? look at pKa... other nucleophiles R C C H H2N Na ? H2N pKa = 35 Na > R C C H pKa = 26 alkynes are useful nucleophiles due to wealth of that are not functional group interconversions enolates... they can undergo can we deprotonate the pKa would them like this? suggest that we can 4 5 6 ©marco belluci@flickr other nucleophiles: alkynes other nucleophiles: nitriles Text R C C H H2N Na R C C Br Ph Na CN Ph Br Ph CN R C C Ph nitriles are good once formed alkynyl anion , why can we readily deprotonate nucleophiles undergoes standard nucleophilic the simplest is cyanide, a alkynes but not alkenes (or long cylinder of charge reactions ? alkanes) think about molecular orbitals 7 8 9
- 2. other nucleophiles: nitriles the use of nitriles... N N O C :base C OH R CN C H Ph H Ph H R OH cyanide has an obvious problem Br Ni / H2 nitriles are Electron withdrawing & allow resonance CN R CN R NH2 stabilisation so are easily nitriles are useful as they deprotonated readily undergo functional Ph group interconversion ©mouse@flickr 10 11 12 mechanism 1: carbonyl without mechanism 2: carbonyl with leaving group leaving group Now, attack on... O O Cnuc O O Cnuc Cnuc Cnuc R1 R2 R1 LG R1 LG O R1 R2 there are two outcomes to attack on if there is a leaving group then carbonyl is reformed & double addition is observed the carbonyl group O HO Cnuc O Cnuc Cnuc this is the if the substrate has no C important one leaving group get simple addition HO Cnuc R1 R2 R1 Cnuc R1 Cnuc R1 Cnuc 13 14 15 Addition of Grignard reagents Addition of Grignard reagents organolithium reagents O HO Rc O HO Rc Rc MgBr Rc Li R1 R2 R1 R2 O HO Rc R1 R2 R1 R2 Rc MgBr R1 OR2 R1 Rc O HO Rc O Rc BrMg H Rc Li R1 R2 addition to an ester always R1 OR2 R1 Rc results in double addition addition to aldehyde/ketone reason: the ketone similar to Grignard first forms an alkoxide, which is intermediate is more reactive reagents except more than the starting material nucleophilic (& BAsic) this can cause then protonated on work-up problems (see later) 16 17 18
- 3. increased reactivity allows... O Me Li O Me Li O Li H O Li R1 O R1 O Li R1 Me deprotonation gives the normally non-electrophilic H2O examples Cl carboxylate anion O OH R1 OH OH Cl R1 Me Me N but reactivity of organolithiums N allows a second attack and a route fenarimol to ketones antifungal ©bmooneyatwork@flickr 19 20 21 organometallic reagent in total C–C bond formation important... synthesis deprotonate methyl group; OMe I can’t find pKa but anywhere from 40 to 25 Li Cl OMe Cl LiNiPr2 HO N3 N N N OH Cl OTBDPS OTBDPS OMe O Cl N N N N N3 OH O N quinine 22 23 24 C–C bond formation important... big OMe OMe N LiNiPr2 HO N3 problem OTBDPS OTBDPS N reactive species is: OMe OMe N3 Li O especially with N N organolithiums ©furryscaly@flickr big problem 25 26 27
- 4. Nucleophilicity versus basicity Nucleophilicity versus basicity transmetallation Li can give a less basic derivative CeCl3 organocerium is less basic so Li Li will perform the desired MeO MeO MeO H H MeO H addition OH Cl2Ce O O O x MeO MeO OH O OMe OMe OMe OMe this is what we get... this is what we want... organolithium reagents are OMe OMe basic & often cause deprotonation not addition 28 29 30 the wittig reaction (or at least one of them) formation of ylide PPh3 Ph3P: R2 Br Br O PPh3 R2 H formation of R2 a ylid (or ylide) is a compound with positive & alkenes R1 negative charges on adjacent base R1 R2 atoms PPh3 PPh3 this is the basic transformation, reaction of an aldehyde/ketone with a R2 R2 phosphorus ylid 31 32 33 formation of ylide two possible mechanisms... second mechanism PPh3 O PPh3 Ph3P R O PPh3 O PPh3 O Ph3P: R2 Br Br R this is the easier of the R2 H mechanisms to understand & is good H H for explaining the stereochemical betaine the phosphorane or ylide better mechanism but some of the (resonance forms of each other) base outcome stereochemical arguments are less is made prior to addition of the it is almost convincing electrophile certainly wrong PPh3 proceeds by a PPh3 PPh3 PPh3 [2+2] cycloaddition O O R2 R2 oxaphosphetane 34 35 36
- 5. Wittig normally gives Z-alkenes Reason: Sterics Reason: Sterics of addition of addition O PPh3 O PPh3 R1 R2 R1 R2 this means that substituents areR1 side once R2 same oxaphosphetane has R1 R2 formed. this step is irreversible so must O PPh3 proceed to cis-alkene even though it is R1 R2 the substituents try to stay as far apart as possible during less stable cycloaddition (or nucleophilic addition R1 R2 Ph3P depending on the mechanism) Ph3P H H O PPh3 O PPh3 O O if R2 is not involved in R1 R1 R2 R1 R1 R2 reaction (non-stabilised ylid) then R2 H the cis alkene is favoured R2 H 37 38 39 Wittig normally gives Z-alkenes Text product natural isolate from bushmint O O PPh3 O EtO O OHC H H O example OAc OAc O O O O OAc OAc EtO O H H O ©cotinis@flickr O anamarine 40 41 42 Stabilised ylides Stabilised ylide normally gives Reason: reversibility of addition E-alkenes O PPh3 slow R1 CO2Et R1 CO2Et O PPh3 O PPh3 O O PPh3 R2 R1 R1 CO2Et R2 PPh3 R2 R2 R1 O PPh3 fast R1 O CO2Et R1 CO2Et if the substituent on the ylid can stabilise anion (EWG or resonance stabilisation) the first step cycloaddition , then we are said to have a stabilised ylid and the mechanism hopefully explains why (or nucleophilic attack) is reversible. The equilibrium the trans-alkene predominates stabilised ylides favour the more stable favours the more stable adduct in which the substituents trans-alkene are as far apart as possible 43 44 45
- 6. Stabilised ylide normally gives E-alkenes O PPh3 EtO EtO2C O OHC O O O this is from a different synthesis of anamarine 46
