Ionic equilibria two
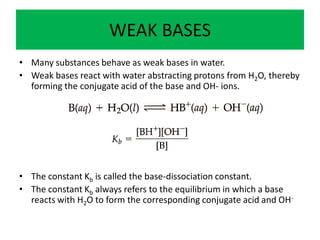
- 1. WEAK BASES • Many substances behave as weak bases in water. • Weak bases react with water abstracting protons from H2O, thereby forming the conjugate acid of the base and OH- ions. • The constant Kb is called the base-dissociation constant. • The constant Kb always refers to the equilibrium in which a base reacts with H2O to form the corresponding conjugate acid and OH-
- 3. EXAMPLES Using Kb to Calculate OH- • Calculate the concentration of OH- in a 0.15 M solution of NH3.
- 4. Because K1 is small, we can neglect the small amount of NH3 that reacts with water, as compared to the total NH3 concentration; that is, we can neglect x relative to 0.15 M.
- 5. RELATIONSHIP BETWEEN Ka AND Kb • Consider the NH4+ and NH3 conjugate acid-base pair and for each of these species reacts with water
- 6. - left with just the autoionization of water.
- 7. • The product of the acid-dissociation constant for an acid and the base-dissociation constant for its conjugate base equals the ion-product constant for water. • As the strength of an acid increases (larger Ka),the strength of its conjugate base must decrease (smaller Kb ) so that the product Ka x Kb equals 1.0 x 10-14 at 25oC. • The Ka and Kb data in Table 16.5 A demonstrate this relationship. • Remember, this important relationship applies only to conjugate acid-base pairs.
- 8. • The values for acid- or base-dissociation constants in a chemistry handbook is expressed as pKa or pKb – that is as -log Ka or -log Kb
- 9. EXAMPLES Calculating Ka or Kb for a Conjugate Acid-Base Pair • Calculate: (a) the base-dissociation constant, Kb , for the fluoride ion (F-) (b) the acid dissociation constant, Ka , for the ammonium ion (NH4+) Ka for the weak acid, HF =6.8 x 10-4 Kb for NH3 = 1.8 x 10-5
- 10. Kb , for the fluoride ion (F-) Ka , for the ammonium ion (NH4+)
- 11. ACID-BASE PROPERTIES OF SALT SOLUTIONS • Ions can also exhibit acidic or basic properties – For example, we calculated Ka for NH4+ and Kb for F- • Such behaviour implies that salt solutions can be acidic or basic. • Nearly all salts are strong electrolytes, so when salts dissolve in water, they are completely dissociated. • Consequently, the acid-base properties of salt solutions are due to the behavior of their constituent cations and anions. • Many ions are able to react with water to generate H+ (aq) or OH- (aq). • This type of reaction is often called hydrolysis. • The pH of an aqueous salt solution can be predicted qualitatively by considering the ions of which the salt is composed.
- 12. An Anion's Ability to React with Water • In general, an anion, X- in solution can be considered the conjugate base of an acid. – Eg Cl- is the conjugate base of HCl – CH3COO- is the conjugate base of CH3COOH. • Whether an anion reacts with water to produce hydroxide depends upon the strength of the acid to which it is conjugate. • To identify the acid and assess its strength, we can simply add a proton to the anion's formula: • If HX is one of the 7 strong acid, then have to tendency to abstract protons from water
- 13. • The anion X- will not affect the pH of the solution. • The presence of Cl- in an aqueous solution,for example, does not result in the production of any OH- and does not affect the pH. – Cl- is a spectator in acid-base chemistry.
- 14. • If HX is not one of the seven strong acids, then it is a weak acid. • In this case, the conjugate base X- is a weak base. • This anion will react to a small extent with water to produce the weak acid and hydroxide ions:
- 15. • The OH- ion generated increases the pH of the solution, making it basic. • Acetate ion (CH3COO-), for example, being the conjugate base of a weak acid, reacts with water to produce acetic acid and hydroxide ions, thereby increasing the pH of the solution. • Anions that still have ionizable protons, such as HSO3- are amphiprotic. – They can act as either acids or bases. • Their behavior toward water will be determined by the relative magnitudes of Ka and Kb for the ion • If Ka > Kb : the ion will cause the solution to be acidic. • If Kb > Ka : the solution will be basic.
- 16. EXAMPLES Predicting Whether the Solution of an Amphiprotic Anion ls Acidic or Basic • Predict whether the salt Na2HPO4 will form an acidic solution or a basic solution on dissolving in water. • This substance is an ionic compound composed of Na+ and HPO42- ions. • Na+ has no influence on pH, merely a spectator ion in acid-base chemistry. • The reaction with the larger equilibrium constant will determine whether the solution is acidic or basic.
- 17. • The value of Ka for Equation 16.45 is 4.2 x 10-13 • Based on • We want to know Kb for the base HPO42- knowing the value of Ka for the conjugate acid H2PO4- • Because Ka for H2PO4- is 6.2 x 10-8, we calculate Kb for HPO42- to be 1.6 x 10-7 . • 105 times larger than Ka for HPO42- thus, the reaction • shown in Equation 16.46 predominates over that in Equation 16.45, and the solution will be basic.
- 18. A Cation's Ability to React with Water • Polyatomic cations whose formulas contain one or more protons can be considered the conjugate acids of weak bases. • NH4+, for example, is the conjugate acid of the weak base NH3. • Thus, NH4+ is a weak acid and will donate a proton to water, producing hydronium ions and thereby lowering the pH
- 19. Combined Effect of Cation and Anion in Solution • If an aqueous salt solution contains an anion that does not react with water and a cation that does not react with water, expect the pH neutral. • If the solution contains an anion that reacts with water to produce hydroxide and a cation that does not react with water, expect the pH basic. • If the solution contains a cation that reacts with water to produce hydronium and an anion that does not react with water, expect the pH acidic. • Finally, a solution may contain an anion and a cation both capable of reacting with water both hydroxide and hydronium will be produced. – Whether the solution is basic, neutral, or acidic will depend upon the relative abilities of the ions to react with water.
- 20. To summarize: 1. An anion that is the conjugate base of a strong acid, for example, Br- will not affect the pH of a solution. (It will be a spectator ion in acid-base chemistry.) 2. An anion that is the conjugate base of a weak acid, for example, CN- will cause an increase in pH. 3. A cation that is the conjugate acid of a weak base, for example, CH3NH3+ will cause a decrease in pH. 4. The cations of group 1A and heavier members of group 2A (Ca2+, Sr2+, and Ba2+) will not affect pH. These are the cations of the strong Arrhenius bases. (They will be spectator ions in acid-base chemistry.) 5. Other metal ions will cause a decrease in pH. 6. When a solution contains both the conjugate base of a weak acid and the conjugate acid of a weak base, the ion with the larger equilibrium constant, Ka or Kb will have the greater inJluence on the pH.
- 21. THE COMMON ION EFFECT • Consider solutions that contain a weak acid, such as acetic acid (CH3COOH), and a soluble salt of that acid such as sodium acetate (CH3COONa). • Notice that two substances that share a common ion CH3COO-. • View these solutions from the perspective of Le Chatelier's principle.
- 22. • Sodium acetate is a soluble ionic compound and is therefore a strong electrolyte. • Consequently, it dissociates completely in aqueous solution to form Na+ and CH3COO- ions: • In contrast, CH3COOH is a weak electrolyte that ionizes as • The CH3COO- from CH3COONa causes this equilibrium to shift to the left, thereby decreasing the equilibrium concentration of H+(aq).
- 23. • Whenever a weak electrolyte and a strong electrolyte contain a common ion, the weak electrolyte ionizes less than it would if it were alone in solution. • This is the common-ion effect.
- 24. BUFFERED SOLUTIONS • Solutions containing a weak conjugate acid- base pair can resist drastic changes in pH upon the addition of small amounts of strong acid or strong base. • Called buffered solutions (or buffers).
- 25. Composition and Action of Buffered Solutions • A buffer resists changes in pH because it contains both an acid to neutralize OH- ions and a base to neutralize H+ ions. • The acid and base that make up the buffer must not consume each other through a neutralization reaction. • These requirements are fulfilled by a weak acid-base conjugate pair such as CH3COOH-CH3COO- or NH4- -NH3 . • Buffers are often prepared by mixing a weak acid or a weak base with a salt of that acid or base. • The CH3COOH-CH3COO- buffer can be prepared: • Eg by adding CH3COONa to a solution of CH3COOH. • The NH4+ -NH3 buffer can be prepared by adding NH4CI to a solution of NH3. • By choosing appropriate components and adjusting their relative concentrations, we can buffer a solution at virtually any pH.
- 26. To understand how a buffer works, consider : • a buffer composed of a weak acid (HX) and one of its salts (MX, where M+ could be Na+ , K+ or another cation). • The acid-dissociation equilibrium in this buffered solution involves both the acid and its conjugate base: • pH is determined by 2 factors: – the value of Ka for the weak-acid component of the buffer and – the ratio of the concentrations of the conjugate acid-base pair, [HX]/ [X-].
- 27. • If OH- ions are added to the buffered solution they react with the acid component of the buffer to produce water and X- • This reaction causes [HX] to decrease and [X-] to increase. • As long as the amounts of HX and X- in the buffer are large compared to the amount of OH- added, however, the ratio [HX]/[X-] does not change much, and so change in pH is small. • If H+ ions are added, they react with the base component of the buffer: • Can be represented also as: • The reaction causes [X- ] to decrease and [HX] to increase. • As long as the change in the ratio [HX]/[X- ] is small, the change in pH will be small.
- 28. Buffer action. • When a small portion of OH- is added to a buffer consisting of a mixture of the weak acid HF and its conjugate base (left), the OH- reacts with the HF decreasing [HF] and increasing [F-] in the buffer. • Conversely, when a small portion of H+ is added to the buffer (right), the H+ reacts with the F-, decreasing [F-] and increasing [HF] in the buffer. • Because pH depends on the ratio of F- to HF, the resulting pH change is small.
- 29. Calculating the pH of a Buffer • From equation • where [acid] and [base] refer to the equilibrium concentrations of the conjugate acid-base pair. • Note that when [base] : [acid], pH : pKa • Equation called the Henderson-Hasselbalch equation
- 30. • In doing equilibrium calculations, we can normally neglect the amounts of the acid and base of the buffer that ionize. • Therefore, we can usually use the starting concentrations of the acid and base components of the buffer directly
- 31. Example What is the pH of a buffer that is 0.12 M in lactic acid [CH3CH(OH)COOH, or HC3H5O3] and 0.10 M in sodium lactate [CH3CH(OH)COONa or NaC3H5O3]? For lactic acid, Ka = 1.4 x 10-4 • Because HC3H5O3 is a weak electrolyte and NaC3H5O3 is a strong electrolyte, the major species in-solution are HC3H5O3, Na+, and C3H5O3- Na+ ion is a spectator ion. • The HC3H5O3 - C3H5O3- conjugate -acid-base pair determines [H+] and thus pH • [H+] can be determined using the acid-dissociation equilibrium of lactic acid
- 32. Because Ko is small and a common ion is present, we expect x to be small relative to either 0.12 or 0.10 M. Thus, our equation can be simplified to give :
- 33. • Could used the Henderson-Hasselbalch equation to calculate pH directly:
- 34. Buffer Capacity and pH Range • Two important characteristics of a buffer • Buffer capacity is the amount of acid or base the buffer can neutralize before the pH begins to change to an appreciable degree • The pH of the buffer depends on the Ka for the acid and on the relative concentrations of the acid and base that comprise the buffer. • Eg: [H+ ] for a 1-L solution that is 1M in CH3COOH and 1 M in CH3COONa will be the same as for a 1-L solution that is 0.1 M in CH3COOH and 0.1 M in CH3COONa • The first solution has a greater buffering capacity, however, because it contains more CH3COOH and CH3COO-. • The greater the amounts of the conjugate acid-base pair, the more resistant is the ratio of their concentrations, and hence the pH, is to change.
- 35. • The pH range of any buffer is the pH range over which the buffer acts effectively. • Buffers most effectively resist a change in pH in either direction when the concentrations of weak acid and conjugate base are about the same.
- 36. EXAMPLES Calculating pH Changes in Buffers A buffer is made by adding 0.300 mol CH3COOH and 0.300 mol CH3COONa to enough water to make 1.00 L of solution. The pH of the buffer is 4.74. Calculate the pH of this solution after 0.020 mol of NaOH is added. Stoichiometry Calculation: •The OH- provided by NaOH reacts with CH3COOH, the weak acid component of the buffer. Prior to this neutralization reaction, there are 0.300 mol each of CH3 COOH and CH3COO - . •Neutralizing the 0.020 mol OH- requires 0.020 mol of CH3COOH. Consequently, the amount of CH3COOH decreased by 0.020 mol, and the amount of the product of the neutralizition, CH3COO- increases by 0.020 mol. •We can create a table to see how the composition of the buffer changes as a result of its reaction with OH-:
- 37. Equilibrium Calculation: We now turn our attention to the equilibrium that will determine the pH of the buffer, namely the ionization of acetic acid. Using the quantities of CH3COOH and CH3COO- remaining in the buffer, we candetermine the pH using the Henderson-Hasselbalch equation.
- 38. If 0.020 mol of H+ as added to the buffer, we would proceed in a similar way to calculate the resulting pH of the buffer. In this case the pH decreases by 0.06 units, giving pH : 4.68
- 39. Calculation of the pH of a buffer after the addltlon of acid or base. •First consider how the neutralization reaction between the added strong acid or strong base and the buffer affects the composition of the buffer (stoichiometry calculation). Then calculate the pH of the remaining buffer (equilibrium calculation). As long as the amount of added acid or base does not exceed the buffer capacity, the Henderson-Hasselbalch equation, can be used for the equilibrium calculation.
