[Infographic] A One Page Guide to Global GDP Guidelines
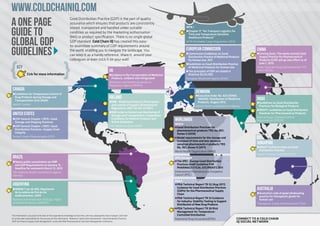
- 1. www.coldchainiq.com A One Page Good Distribution Practice (GDP) is the part of quality assurance which ensures that products are consistently Guide to stored, transported and handled under suitable IATA v condition as required by the marketing authorisation r Chapter 17 “Air Transport Logistics for Time and Temperature Sensitive (MA) or product specification. There is no single global Global GDP Healthcare Products” GDP standard. Cold Chain IQ has created this easy- IATA Perishable Cargo Regulations (PCR) to-assimilate summary of GDP requirements around Guidelines the world, enabling you to navigate the landscape. You can keep it as a handy reference, share it around your colleagues or even stick it on your wall! european commission r Commission Guidelines on Good Distribution Practice of Medicinal Products china r Coming Soon: The newly revised Good Supply Practice for Pharmaceutical Products (GSP) will go into effect as of for Human Use, 2011 r Guidelines on Good Distribution Practice June 1, 2013 KEy of Medicinal Products for Human Use State Food and Drug Administration, P.R. uk r The principles of GDP are stated in China (SFDA) Cick for more information r Guidance in the Transportation of Medicinal Directive 92/25/EEC Products, ambient and refrigerated European Medicines Agency (EMA) Medicines and Healthcare products Regulatory Agency (MHRA) canada r Guidelines for Temperature Control of denmark r Executive Order No. 823 (IDRAC Drug Products during Storage and ireland 148449): Distribution of Medicinal India Transportation (GUI-0069) r IMB - Medicinal Products (Prescription Products, August 2012 r Guidelines on Good Distribution Health Canada and Control of Supply) (Amendment) Danish Health and Medicines Agency Practices for Biological Products Regulations 2007 (SI 201 of 2007) r DRAFT: Guidelines on Good Distribution r IMB Guide to Control and Monitoring of united states Storage and Transportation Temperature Practices for Pharmaceutical Products Central Drugs Standard Control r USP General Chapter <1079> Good Storage and Shipping Practices Conditions for Medical Products and Active Substance Worldwide Organization (CDSCO) WHO v r USP General Chapter <1083> Good Irish Medicines Board (IMB) r Good Distribution Practices for Distribution Practices—Supply Chain pharmaceutical products TRS No. 957, Integrity Annex 5 (2010) United States Pharmacopeia (USP) r Model requirements for the storage and transport of time and tem-perature sensitive pharmaceutical products TRS singapore No. 961, Annex 9 (2011) r DRAFT Guidance notes on Good World Health Organization (WHO) Distribution Practice Health Sciences Authority (HSA) Brazil IPEC Europe v r Opens public consultation on GMP r The IPEC –Europe Good Distribution and GDP Requirements on January 15. Practices Audit Guideline FOR Deadline for comments March 12, 2013 PHARMACEUTICAL EXCIPIENTS 2011 The National Health Surveillance Agency International Pharmaceutical Excipients (Anvisa) Council (IPEC) PDA v Argentina r PDA Technical Report TR 52 (Aug 2011) r ANMAT Ley 26.492, Regulación Guidance for Good Distribution Practices (GDPs) for the Pharmaceutical Supply Australia de la cadena de frío de los r Australian code of good wholesaling Chain medicamentos, 2009 practice for therapeutic goods for r PDA Technical Report TR 53 Guidance National Administration of Drugs, Foods human use for Industry: Stability Testing to Support and Medical Devices (ANMAT) Therapeutic Goods Administration (TGA) Distribution of New Drug Products r PDA Technical Report TR 58 Risk Management for Temperature- This information is accurate to the best of the respondents knowledge at that time, and may subsequently have changed. Cold Chain Controlled Distribution IQ cannot take responsibility for the accuracy of this information. Reference: David Ulrich presentation “Good Distribution Practices Parenteral Drug Association(PDA) Connect to a cold chain (GDP‘s) & Pharma Supply Chain Management” at the 2011 PDA Pharmaceutical Cold Chain Management Conference. IQ social network
