C20 Review Unit 02 Chemical Reactions
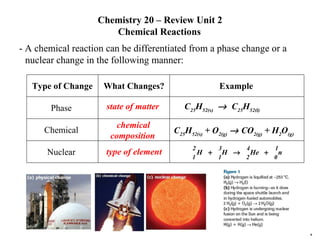
- 1. Chemistry 20 – Review Unit 2 Chemical Reactions - A chemical reaction can be differentiated from a phase change or a nuclear change in the following manner: state of matter C 25 H 52(s) C 25 H 52(l) chemical composition C 25 H 52(s) + O 2(g) CO 2(g) + H 2 O (g) type of element * Type of Change What Changes? Example Phase Chemical Nuclear
- 2. - In all chemical reactions, you start with one or more substances called reactants and produce substances called products . - The plus symbol ( + ) is used to separate lists of chemicals and is interpreted as the word “ and ”. - Reactants and products are separated by an arrow ( ) and is interpreted as the word “ yields ”. - How do chemists recognize that a chemical reaction has taken place? What is the evidence for a chemical reaction ? *
- 3. 1) Energy Exchange – 1) Energy Exchange – heat is given off ( exothermic reaction) or heat is absorbed by the reactants ( endothermic reaction) Examples: - the carbon in wood burns, releasing energy in the form of light and heat, as products are made - C (s) + O 2(g) CO 2(g) + heat + light - exothermic reaction - electricity ( energy ) is added to water in a process called electrolysis - 2 H 2 O (l) + energy 2 H 2(g) + O 2(g) - endothermic reaction *
- 4. 2) Gas ( bubbles ) Produced – sometimes bubbling can be easily noticed ( fizzing ); at other times a vapour is produced ( smoke ) Examples: - an acid is poured into a metal - Zn (s) + HCl (aq) ZnCl 2(aq) + H 2(g) - baking soda fizzes in water - NaHCO 3(s) + H 2 O (l) CO 2(g) + NaOH (aq) 3) Formation of a Precipitate – a precipitate is a solid , usually formed by mixing two clear solutions Examples: - two clear solutions are mixed and a yellow solid forms - 2 NaI (aq) + Pb(NO 3 ) 2(aq) 2 NaNO 3(aq) + PbI 2(s) - iron rusts when left out in the rain - 2 Fe (s) + 2 H 2 O (l) + O 2(g) 2 Fe(OH) 2(s) *
- 5. 4) Colour Change – a substance formed may exhibit a new characteristic colour Examples: - when nitric acid reacts with copper metal, a dark brown gas is produced and the resulting solution turns blue - Cu (s) + HNO 3(aq) NO 2(g) + Cu(NO 3 ) 2(aq) + H 2 O (l) Note: This equation is not balanced. Technically, the equation is written differently in Chemistry 30, but for illustrative purposes, we’ll use it in this form. *
- 6. Types of Chemical Reactions Simple Composition - Just as the name implies, this reaction is the simplest of all. It implies that a product is composed from the simplest substances . - A generic equation can be used to describe a simple composition reaction. - Remember that reactants are placed on the left side of the equation and the products are found on the right side. - element + element compound * any number of elements only one compound formed
- 7. hydrogen and oxygen yields water vapour H 2(g) + O 2(g) H 2 O (g) - Is this equation correct? What is missing? - Whenever we write out a chemical formula, we must make sure that the equation is balanced . - The number of atoms of each element must be the same number on both reactant and product sides. - To finish balancing the equation: H 2(g) + O 2(g) H 2 O (g) - There are 2 hydrogens on the left and 2 hydrogens on the right, therefore the hydrogens are balanced . * Examples 1) Word Equation Chemical Equation (Formula)
- 8. H 2(g) + O 2(g) H 2 O (g) - There are 2 oxygens on the left but 1 oxygen on the right. - To balance the oxygens we place a number in front of water to make the oxygens balance. The number being placed is called a coefficient . - We never place a subscript on atoms to balance, since subscripts would change the chemical composition of a reactant or product. 2 H 2(g) + O 2(g) 2 H 2 O (g) and not H 2(g) + O 2(g) H 2 O 2(g) * Note that the chemical composition of water has been changed so that we do not have water any more but another chemical called hydrogen peroxide.
- 9. calcium and chlorine yield calcium chloride Ca (s) + Cl 2(g) CaCl 2(s) magnesium and oxygen yield magnesium oxide Mg (s) + O 2(g) MgO (s) Mg (s) + O 2(g) 2 MgO (s) 2 Mg (s) + O 2(g) 2 MgO (s) aluminum and sulfur yield aluminum sulfide Al (s) + S 8(s) Al 2 S 3(s) Al (s) + S 8(s) 8 Al 2 S 3(s) 16 Al (s) + S 8(s) 8 Al 2 S 3(s) 16 Al (s) + 3 S 8(s) 8 Al 2 S 3(s) * 2) Word Equation Chemical Formula 3) Word Equation Chemical Formula 4) Word Equation Chemical Formula
- 10. Simple Decomposition - Simple decomposition is exactly the same as simple composition except that it is the reverse equation. - The generic equation can be stated as follows: compound element + element + … Examples 1) Potassium permanganate is decomposed. KMnO 4(s) K (s) + Mn (s) + O 2(g) KMnO 4(s) K (s) + Mn (s) + 2 O 2(g) 2) Ammonia is decomposed. NH 3(g) N 2(g) + H 2(g) NH 3(g) N 2(g) + 3 H 2(g) 2 NH 3(g) N 2(g) + 3 H 2(g) 3) Calcium carbonate is decomposed. CaCO 3(s) Ca (s) + C (s) + O 2(g) CaCO 3(s) Ca (s) + C (s) + 3 O 2(g) 2 CaCO 3(s) Ca (s) + C (s) + 3 O 2(g) *
- 11. 4) Sucrose is decomposed. C 12 H 22 O 11(s) C (s) + H 2(g) + O 2(g) C 12 H 22 O 11(s) 12 C (s) + H 2(g) + O 2(g) C 12 H 22 O 11(s) 12 C (s) + 11 H 2(g) + O 2(g) C 12 H 22 O 11(s) 12 C (s) + 11 H 2(g) + 5.5 O 2(g) 2 C 12 H 22 O 11(s) 24 C (s) + 22 H 2(g) + 11 O 2(g) *
- 12. Single Replacement - As the name implies, something that is single is replaced . The generic equation is: element + compound element new + compound new Na (s) Na (s) + LiCl (aq) Na (s) + LiCl (aq) Li (s) Na (s) + LiCl (aq) Li (s) + NaCl (aq) * Example 1) sodium metal and a lithium chloride solution yield lithium metal and a sodium chloride solution
- 13. How does this work? - Break up each atom to show what their ions look like. - Replace positive or negative atoms (switch) and reform new products. Na (s) + LiCl (aq) ? + ? Na + Na + Li + Cl Na + Li + Cl Li + Na + Cl Na (s) + Na (s) + LiCl (aq) Na (s) + LiCl (aq) Li (s) + Na (s) + LiCl (aq) Li (s) + NaCl (aq) *
- 14. Double Replacement - In double replacement reactions, two compounds are reacted to form two new compounds . - Generic formula: compound 1 + compound 2 compound 3 + compound 4 Examples 1) Sodium chloride solution and silver nitrate solutions are mixed. NaCl (aq) + NaCl (aq) + AgNO 3(aq) NaCl (aq) + AgNO 3(aq) ? + ? Na + Cl Na + Cl Ag + Na + Cl Ag + NO 3 Na + Cl Ag + NO 3 forms new products Na + Cl Ag + NO 3 Na + NO 3 + Na + Cl Ag + NO 3 Na + NO 3 + Ag + Cl - NaCl (aq) + AgNO 3(aq) NaNO 3(aq) + AgCl (s) * join join
- 15. 2) Solutions of lead (II) nitrate and sodium iodide are mixed. Pb ( NO 3 ) 2(aq) + Pb ( NO 3 ) 2(aq) + NaI (aq) Pb ( NO 3 ) 2(aq) + NaI (aq) ? + ? Pb 2+ NO 3 – + Pb 2+ NO 3 – + Na + I – Pb 2+ NO 3 – + Na + I – Pb 2+ I – + Pb 2+ NO 3 – + Na + I – Pb 2+ I – + Na + NO 3 – Pb ( NO 3 ) 2(aq) + Pb ( NO 3 ) 2(aq) + NaI (aq) Pb ( NO 3 ) 2(aq) + NaI (aq) PbI 2(s) + Pb ( NO 3 ) 2(aq) + NaI (aq) PbI 2(s) + NaNO 3(aq) Pb ( NO 3 ) 2(aq) + NaI (aq) PbI 2(s) + 2 NaNO 3(aq) Pb ( NO 3 ) 2(aq) + 2 NaI (aq) PbI 2(s) + 2 NaNO 3(aq) *
- 16. 3) Solutions of gallium chloride and hydrosulfuric acid are mixed. GaCl 3(aq) + GaCl 3(aq) + H 2 S (aq) ? + ? Ga 3+ Cl – + Ga 3+ Cl – + H + S 2– Ga 3+ Cl – + H + S 2– Ga 3+ S 2– + Ga 3+ Cl – + H + S 2– Ga 3+ S 2– + H + Cl – GaCl 3(aq) + GaCl 3(aq) + H 2 S (aq) GaCl 3(aq) + H 2 S (aq) Ga 2 S 3(aq) + GaCl 3(aq) + H 2 S (aq) Ga 2 S 3(aq) + HCl (aq) _ GaCl 3(aq) + _ H 2 S (aq) _ Ga 2 S 3(aq) + _ HCl (aq) _ GaCl 3(aq) + 3 H 2 S (aq) _ Ga 2 S 3(aq) + _ HCl (aq) _ GaCl 3(aq) + _ H 2 S (aq) _ Ga 2 S 3(aq) + 6 HCl (aq) 2 GaCl 3(aq) + _ H 2 S (aq) _ Ga 2 S 3(aq) + _ HCl (aq) _ GaCl 3(aq) + _ H 2 S (aq) 1 Ga 2 S 3(aq) + _ HCl (aq) *
- 17. 4) Solutions of calcium nitrate and sodium phosphate are mixed. Ca(NO 3 ) 2(aq) + Na 3 PO 4(aq) ? + ? Ca 2+ NO 3 – + Ca 2+ NO 3 – + Na + PO 4 3– Ca 2+ NO 3 – + Na + PO 4 3– Ca 2+ PO 4 3– + Ca 2+ NO 3 – + Na + PO 4 3– Ca 2+ PO 4 3– + Na + NO 3 – Ca ( NO 3 ) 2(aq) + Ca ( NO 3 ) 2(aq) + Na 3 PO 4(aq) Ca ( NO 3 ) 2(aq) + Na 3 PO 4(aq) Ca 3 ( PO 4 ) 2(aq) + Ca ( NO 3 ) 2(aq) + Na 3 PO 4(aq) Ca 3 ( PO 4 ) 2(aq) + NaNO 3(aq) _ Ca ( NO 3 ) 2(aq) + _ Na 3 PO 4(aq) _ Ca 3 ( PO 4 ) 2(s) + _ NaNO 3(aq) 3 Ca ( NO 3 ) 2(aq) + _ Na 3 PO 4(aq) _ Ca 3 ( PO 4 ) 2(s) + _ NaNO 3(aq) _ Ca ( NO 3 ) 2(aq) + _ Na 3 PO 4(aq) _ Ca 3 ( PO 4 ) 2(s) + 6 NaNO 3(aq) _ Ca ( NO 3 ) 2(aq) + 2 Na 3 PO 4(aq) _ Ca 3 ( PO 4 ) 2(s) + _ NaNO 3(aq) _ Ca ( NO 3 ) 2(aq) + _ Na 3 PO 4(aq) 1 Ca 3 ( PO 4 ) 2(s) + _ NaNO 3(aq) *
- 18. Hydrocarbon Combustion In hydrocarbon combustion, a fuel made up of at least hydrogen and carbon , is burned ( combustion ) in the presence of oxygen . Generic Formula: hydrocarbon + hydrocarbon + O 2(g) hydrocarbon + O 2(g) CO 2(g) + hydrocarbon + O 2(g) CO 2(g) + H 2 O (g) Examples 1) Methane is burned to heat our homes in winter. CH 4(g) + CH 4(g) + O 2(g) CH 4(g) + O 2(g) CO 2(g) + CH 4(g) + O 2(g) CO 2(g) + H 2 O (g) _ CH 4(g) + _ O 2(g) _ CO 2(g) + _ H 2 O (g) _ CH 4(g) + _ O 2(g) 1 CO 2(g) + _ H 2 O (g) _ CH 4(g) + _ O 2(g) _ CO 2(g) + 2 H 2 O (g) _ CH 4(g) + 2 O 2(g) _ CO 2(g) + _ H 2 O (g) *
- 19. 2) Gasoline is combusted in a car engine. C 8 H 18(l) + C 8 H 18(l) + O 2(g) CO 2(g) + H 2 O (g) _ C 8 H 18(l) + _ O 2(g) _ CO 2(g) + _ H 2 O (g) _ C 8 H 18(l) + _ O 2(g) 8 CO 2(g) + _ H 2 O (g) _ C 8 H 18(l) + _ O 2(g) _ CO 2(g) + 9 H 2 O (g) 2 C 8 H 18(l) + 25 O 2(g) 16 CO 2(g) + 18 H 2 O (g) 3) The burning of wood alcohol produces a flame. CH 3 OH (l) + CH 3 OH (l) + O 2(g) CO 2(g) + H 2 O (g) _ CH 3 OH (l) + _ O 2(g) _ CO 2(g) + _ H 2 O (g) _ CH 3 OH (l) + _ O 2(g) 1 CO 2(g) + _ H 2 O (g) _ CH 3 OH (l) + _ O 2(g) _ CO 2(g) + 2 H 2 O (g) 2 CH 3 OH (l) + 3 O 2(g) 2 CO 2(g) + 4 H 2 O (g) *
- 20. 4) We burn glucose in our body cells. C 6 H 12 O 6(s) + C 6 H 12 O 6(s) + O 2(g) CO 2(g) + H 2 O (g) _ C 6 H 12 O 6(s) + _ O 2(g) _ CO 2(g) + _ H 2 O (g) _ C 6 H 12 O 6(s) + _ O 2(g) 6 CO 2(g) + _ H 2 O (g) _ C 6 H 12 O 6(s) + _ O 2(g) _ CO 2(g) + 6 H 2 O (g) _ C 6 H 12 O 6(s) + 6 O 2(g) _ CO 2(g) + _ H 2 O (g) Other Some chemical reactions do not fit in any of the previously studied chemical reactions. These reactions will then be placed in a category called “ other ”. Examples 1) sulfur trioxide and water sulfuric acid SO 3(g) + SO 3(g) + H 2 O (l) SO 3(g) + H 2 O (l) H 2 SO 4(aq) *
- 21. 2) Nitrogen monoxide gas reacts with oxygen to produce nitrogen dioxide gas. NO (g) + NO (g) + O 2(g) NO (g) + O 2(g) NO 2(g) _ NO (g) + _ O 2(g) _ NO 2(g) 2 NO (g) + _ O 2(g) _ NO 2(g) _ NO (g) + _ O 2(g) 2 NO 2(g) 3) A sodium bicarbonate solution decomposes into a sodium carbonate solution, carbon dioxide and water. NaHCO 3(aq) NaHCO 3(aq) Na 2 CO 3(aq) + NaHCO 3(aq) Na 2 CO 3(aq) + CO 2(g) + NaHCO 3(aq) Na 2 CO 3(aq) + CO 2(g) + H 2 O (l) _ NaHCO 3(aq) _ Na 2 CO 3(aq) + _ CO 2(g) + _ H 2 O (l) 2 NaHCO 3(aq) _ Na 2 CO 3(aq) + _ CO 2(g) + _ H 2 O (l) *
- 22. Determining Whether a Substance Dissolves Easily In Water or Forms a Precipitate (Solid) - We are told that ionic substances are solids at room temperature and molecular substances can be any state depending on which ones we are talking about. - What happens if we try to dissolve them in water? Will they easily dissolve or will they tend not to dissolve, thereby remaining a solid ? - In chemical reactions where solutions are involved, it is necessary to know if a substance dissolves, so that we can place ( aq ) after its chemical formula. - To determine the “ solubility ” of a molecular substance we will need to memorize which ones easily dissolve and which ones don’t. - To determine the “ solubility ” of an ionic substance we will use the “ Solubility Table ” found at the bottom of the Periodic Table of Ions Sheet. *
- 23. - First, find the ions that make up the compound. [ Na + and Cl – ] - Next, locate one of those ions in the top row. [ Na + is a Gr IA ion ] - What does “ All ” and “ None ” mean? - Any Gr IA ion combined with “All” other negative ions will be “ Soluble ”, therefore we we put ( aq ) after that compound’s chemical formula. [ NaCl (aq) ] Which of the following ionic compounds will be soluble? a) ammonium hydroxide b) calcium phosphate c) potassium hydroxide d) strontium carbonate e) silver chloride NH 4 OH (aq) Ca 3 (PO 4 ) 2(s) KOH (aq) SrCO 3(s) AgCl (s) * How does this work? - Suppose you want to know if an ionic substance such as salt, NaCl (s) , dissolves in water.
- 24. _ NaI (aq) + _ NaI (aq) + _ Pb(NO 3 ) 2(aq) _ NaI (aq) + _ Pb(NO 3 ) 2(aq) _ NaNO 3(aq) + _ NaI (aq) + _ Pb(NO 3 ) 2(aq) _ NaNO 3(aq) + _ PbI 2(s) 2 NaI (aq) + _ Pb(NO 3 ) 2(aq) _ NaNO 3(aq) + _ PbI 2(s) _ NaI (aq) + _ Pb(NO 3 ) 2(aq) 2 NaNO 3(aq) + _ PbI 2(s) 2) Crystals of silver nitrate and sodium chloride are placed in separate beakers containing distilled water. Then their contents are mixed. Write out the balanced chemical equation. _ AgNO 3(aq) + _ AgNO 3(aq) + _ NaCl (aq) _ AgNO 3(aq) + _ NaCl (aq) _ AgCl (s) + _ AgNO 3(aq) + _ NaCl (aq) _ AgCl (s) + _ NaNO 3(aq) * Predict the states of matter for each of the following chemical reactions. 1) Crystals of sodium iodide and lead (II) nitrate are placed in separate beakers containing distilled water. Then their contents are mixed. Write out the balanced chemical equation.
- 25. Solubility Table For Ionic Substances * Ion H + Gr IA NH 4 + NO 3 – CH 3 COO – Cl – Br – I – SO 4 2– S 2– OH – PO 4 3 – SO 3 2 – CO 3 2 – Soluble (aq) All All All All All Most Most Gr IA Gr IIA NH 4 + Gr IA NH 4 + Sr 2+ Ba 2+ Gr IA NH 4 + Low Solubility (s) None None None None None Ag + Pb 2+ Hg + Cu + Ag + Pb 2+ Ca 2+ Ba 2+ Sr 2+ Most Most Most
- 26. The Mole Before we discuss the concept of the “mole”, it is important to be able to work with numbers. Scientific Notation Example: Convert 4638600000 into scientific notation 4.638600000 x 10 9 Example: Convert 0.00000475 into scientific notation 4.75 x 10 –6 Note: A positive exponent indicates a number greater than 1 and a negative exponent indicates a number smaller than 1. Example: Calculate the following. a) (4.50 x 10 3 ) (9.25 x 10 5 ) = b) (3.36 x 10 –7 ) (5.50 x 10 4 ) = 4.16 x 10 9 1.85 x 10 –2 9.38 c)
- 27. 6.92 x 10 –4 4.00 x 10 9 4.30 x 10 19 Significant Digits - The number 354 has _ significant digits. - The number 354 has 3 significant digits. - The number 35.4 has _ significant digits. - The number 35.4 has 3 significant digits. - The number 52 has _ significant digits. - The number 52 has 2 significant digits. - The number 3.54 x 10 4 has _ significant digits. - The number 3.54 x 10 4 has 3 significant digits. - The number 0.354 has _ significant digits. - The number 0.354 has 3 significant digits. - The number 0.00354 has _ significant digits. - The number 0.00354 has 3 significant digits. - The number 35400 has _ significant digits. - The number 35400 has 5 significant digits. - The number 0.03540 has _ significant digits. - The number 0.03540 has 4 significant digits. - The number 0.00350040 has _ significant digits. - The number 0.00350040 has 6 significant digits. d) e) = f) =
- 28. Rules For Multiplying and Dividing - When multiplying or dividing, the answer must contain the least number of significant digits that are found in the numbers being calculated . - Example: 3.3 x 0.134 = - The answer is 0.4422 but since the least number of significant digits being used is “ _ ”, the answer must be rounded off to “ _ ” digits. - The answer is 0.4422 but since the least number of significant digits being used is “ 2 ”, the answer must be rounded off to “ 2 ” digits. Answer: Answer: 0.44 - Example: 3746 x 0.120 = - The calculator answer is 449.52 , but since the least number of significant digits is “ _ ”, the answer is rounded off to _ digits. - The calculator answer is 449.52 , but since the least number of significant digits is “ 3 ”, the answer is rounded off to 3 digits. - Example: 3746 x 120 = - The calculator answer is 449520 , but since the least number of significant digits is “ _ ”, the answer is rounded off to _ digits. - The calculator answer is 449520 , but since the least number of significant digits is “ 3 ”, the answer is rounded off to 3 digits. - The answer must be about 450000, but that number has _ digits. - The answer must be about 450000, but that number has 6 digits. - In this case, use scientific notation . Answer: Answer: 4.50 x 10 5 Answer: Answer: 450
- 29. Rules for Adding and Subtracting - Perform the calculation in your calculator. - Determine the least number of place holding there are after the decimal in each entry . - Your answer must contain the least number of place holdings after the decimal . - Round off the answer. Example: 34.65 + 4.235 = - Calculator answer is - Calculator answer is 38.885 . - The least number of place holding after the decimal is “ _ ”. - The least number of place holding after the decimal is “ 2 ”. Answer: Answer: 38.89 - Example: 1356.245 + 245.33 – 0.0001 = 1601.5749 = 1601.5749 = 1601.57 Note: When calculating long series of numbers, round off only at the end .
- 30. The Mole - Atoms are extremely small so it becomes very difficult to make measurements when only a few atoms or molecules are used. - If we wanted to take the mass of 1 atom, it would be appropriate to find the mass of say, 1000 atoms and then divide by 1000, but 1000 atoms still gives us a very small mass to measure . - What number of atoms or molecules would be large enough? 1 dozen? 1 gross (144)? 1 million? - In the early 1800’s, a scientist named Amedeo Avogadro devised a way of measuring the masses of very small particles of matter. - Avogadro suggested that if you took exactly 12.00 g of the carbon-12 atom you would have a very large number of carbon atoms. Just as words like “dozen”, “gross” or “couple” have numerical meaning, he needed a word to describe how many atoms there would be in 12.00 g of carbon-12. - This word is now known as the “ mole ”.
- 31. - One dozen atoms would be 12 atoms. - One gross of atoms would be 144 atoms. - One mole of atoms is 6.02 x 10 23 atoms , now known as Avogadro’s number .
- 32. The Green Pea Analogy (from Alchem 2000) - One hundred (10 2 ) green peas have a volume of about 20 cm 3 or 20 mL in a graduated cylinder. - One million (10 6 ) green peas have enough volume to fill an ordinary refrigerator. - One billion (10 9 ) green peas can fill an average 3 bedroom house. - One trillion (10 12 ) green peas can fill 1000 average homes. - One quadrillion (10 15 ) green peas can fill all the building in a larger city such as Edmonton. - Imagine all of Alberta covered one metre deep in green peas. Now you got one quintillion (10 18 ) green peas. - Imagine that all the continents are now covered 1 metre deep with green peas. Now you have one sextillion (10 21 ). - If you froze all the oceans and totally covered the whole earth with green peas and had 250 planets just like earth, all covered with green peas, you would now have a mole’s worth ! - 250 000 planets like earth, all covered one metre deep in green peas would give you a cotillion (10 27 ), the number of atoms in your body!
- 34. Calculating Number of Particles In Given Number of Moles Examples: 1. How many atoms are there in 2.50 mol of lithium? Since 1 mol of Li (s) = Since 1 mol of Li (s) = 6.02 x 10 23 atoms Since 1 mol of Li (s) = 6.02 x 10 23 atoms then 2.50 mol of Li (s) should have 2.50 mol x 6.02 x 10 23 atoms = Since 1 mol of Li (s) = 6.02 x 10 23 atoms then 2.50 mol of Li (s) should have 2.50 mol x 6.02 x 10 23 atoms = 1.51 x 10 24 atoms or
- 35. 2. How many molecules are there in 25.0 mol of water? 3. How many moles are there in 4.55 x 10 15 atoms of zinc?
- 36. 4. How many moles are there in 2.75 x 10 24 molecules of ammonia?
- 37. Calculating the Molar Mass of Any Substance Find the molar masses for each of the following examples: 1) Cu (s) Since the formula suggests that copper is made up only one type of atom, we simply look up the molar mass value from the periodic table. Cu (s) = Cu (s) = 63.55 g in one mole 2) H 2 O (l) There are 2 hydrogens and 1 oxygen involved in making the water molecule we need to add the molar masses of all atoms. 2 – H = 2 – H = 2 x 1.01 = 2 – H = 2 x 1.01 = 2.02 g 1 – O = 1 – O = 1 x 16.00 = 1 – O = 1 x 16.00 = 16.00 g H 2 O (l) = = 63.55 H 2 O (l) = 18.02
- 38. 3) CaSO 4(s) 1 – Ca = 1 – Ca = 1 x 40.08 = 1 – Ca = 1 x 40.08 = 40.08 g 1 – S = 1 – S = 1 x 32.06 = 1 – S = 1 x 32.06 = 32.06 g 4 – O = 4 – O = 4 x 16.00 = 4 – O = 4 x 16.00 = 64.00 g CaSO 4(s) = 4) Al(OH) 3(s) 1 – Al = 1 – Al = 1 x 26.98 = 1 – Al = 1 x 26.98 = 26.98 g 3 – O = 3 – O = 3 x 16.00 = 3 – O = 3 x 16.00 = 48.00 g 3 – H = 3 – H = 3 x 1.01 = 3 – H = 3 x 1.01 = 3.03 g Al(OH) 3(s) = CaSO 4(s) = 136.14 Al(OH) 3(s) = 78.01
- 39. 5) Na 2 H 2 PO 4(s) 2 – Na = 2 – Na = 2 x 22.99 = 2 – Na = 2 x 22.99 = 45.98 g 2 – H = 2 – H = 2 x 1.01 = 2 – H = 2 x 1.01 = 2.02 g 1 – P = 1 – P = 1 x 30.97 = 1 – P = 1 x 30.97 = 30.97 g 4 – O = 4 – O = 4 x 16.00 = 4 – O = 4 x 16.00 = 64.00 g Na 2 H 2 PO 4(s) = Na 2 H 2 PO 4(s) = 142.97
- 40. Calculations Converting Mass to Moles We can use a simple formula for this type of calculation: - molar mass of water = Examples 1. How many moles are there in 75.0 g of water?
- 41. 2. How many moles are there in 100 g of sucrose? - molar mass of sucrose = Calculations Converting Moles to Mass We can use a simple formula for this type of calculation:
- 42. 2. What is the mass of 25.0 mol of ammonia? Examples 1. What mass do you have in 2.83 mol of table salt?