Endothermic and exothermic process (Data Logging)
•Transferir como PPT, PDF•
0 gostou•2,334 visualizações
Denunciar
Compartilhar
Denunciar
Compartilhar
Recomendados
Mais conteúdo relacionado
Mais procurados
Mais procurados (20)
A case study on Process Condensate Stripper in Ammonia Plant by Prem Baboo.pdf

A case study on Process Condensate Stripper in Ammonia Plant by Prem Baboo.pdf
Transport processes and unit operations geankoplis

Transport processes and unit operations geankoplis
Destaque
Destaque (19)
Endothermic and Exothermic Reactions Lesson PowerPoint

Endothermic and Exothermic Reactions Lesson PowerPoint
Semelhante a Endothermic and exothermic process (Data Logging)
Semelhante a Endothermic and exothermic process (Data Logging) (20)
Chemistry_Energetics_Lecture_1.Chemistry_Energetics_Lecture

Chemistry_Energetics_Lecture_1.Chemistry_Energetics_Lecture
Notes for Unit 17 of AP Chemistry (Thermodynamics)

Notes for Unit 17 of AP Chemistry (Thermodynamics)
Endothermic and exothermic process (Data Logging)
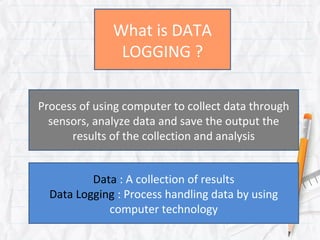
- 1. What is DATA LOGGING ? Process of using computer to collect data through sensors, analyze data and save the output the results of the collection and analysis Data : A collection of results Data Logging : Process handling data by using computer technology
- 2. ELEMENTS OF DATA LOGGING
- 4. Endothermic and exothermic process
- 5. engage a b
- 7. Empower Sensor Data collector
- 9. Result Solution of copper (II) sulphate,
- 11. Solution of anhydrous copper (II) sulphate
- 13. • Is the enthalpy change for this reaction exothermic or endothermic? • What sign should the enthalpy change have? • What is the equation for this reaction? • What bonds are being broken and formed in this reaction? • What is this enthalpy change called?
- 14. • How can we make a triangle between these reactions?
- 15. Enhance Cold packs and putting ice in towel causes a cooling effect on their person’s head and temporarily relieve the pain and fever. Explain.
- 16. a) Cold Pack • When the cold pack is used, the chemicals inside the pack are made to react with each other and this reaction is highly endothermic in nature. • Endothermic reactions involve the absorption of heat.
- 17. • The ammonium nitrate mixing with the water creates cold. The temperature of cold packs can reach back to normal temperature. • The heat energy is taken into the system from the surrounding. The surrounding in this case is the person’s head.
- 18. Extension Production of Ammonia Gas : N2 (g) + 3H2 (g) ↔ 2NH3 (g) What will happen to the reaction if we increase the concentration the temperature of the reactant mixture?
- 19. • If the temperature of a reaction mixture is increase, the equilibrium will shift to decrease the temperature. • Based on Le Chatelier’s Principle which stated that if a chemical system at equilibrium experiences a change in concentration, temperature or total pressure, the equilibrium will shift in order to minimize that changes a new equilibrium is established.
- 20. • So, if we increase the temperature, the equilibrium will shift to the reactant part which is left. • So, the reaction will undergo endothermic reaction as it use up heat energy. • Ammonia will broken down into hydrogen and nitrogen gas. An increase in temperature will decrease the yield of ammonia , NH3.