Pharmacovigilance Expertise Summary for Startups and Strategic Process Improvement
•
3 gostaram•258 visualizações
Denunciar
Compartilhar
Denunciar
Compartilhar
Baixar para ler offline
Recomendados
Implementing MDSAP (Medical Device Single Audit Program) for Compliance Success

Implementing MDSAP (Medical Device Single Audit Program) for Compliance SuccessGlobalCompliancePanel
Mais conteúdo relacionado
Mais procurados
Implementing MDSAP (Medical Device Single Audit Program) for Compliance Success

Implementing MDSAP (Medical Device Single Audit Program) for Compliance SuccessGlobalCompliancePanel
Mais procurados (20)
2nd exl Quality Oversight Conf Szpindor In Process Vendor Audit

2nd exl Quality Oversight Conf Szpindor In Process Vendor Audit
Risk Based Monitoring in Clinical Trials - Impact on Sites

Risk Based Monitoring in Clinical Trials - Impact on Sites
The World Congress Summit on Risk-Based Monitoring and the Quality Risk Manag...

The World Congress Summit on Risk-Based Monitoring and the Quality Risk Manag...
3 Reasons Intelligent Monitoring Outperforms Risk-Based Monitoring

3 Reasons Intelligent Monitoring Outperforms Risk-Based Monitoring
Expanding Patient Enrollment and Site Activation Study Case Study: Partnershi...

Expanding Patient Enrollment and Site Activation Study Case Study: Partnershi...
e-Zest Solutions Inc. - Testing (Healthcare Domain) Competency

e-Zest Solutions Inc. - Testing (Healthcare Domain) Competency
Implementing MDSAP (Medical Device Single Audit Program) for Compliance Success

Implementing MDSAP (Medical Device Single Audit Program) for Compliance Success
Destaque
Destaque (20)
J manning potts-selecoes_das_cartas_de_joao_wesley (4)

J manning potts-selecoes_das_cartas_de_joao_wesley (4)
The suspicious finger of fake wine points to whom?是谁打翻了波尔多的“醋”

The suspicious finger of fake wine points to whom?是谁打翻了波尔多的“醋”
Lightspeed Connect - Niels de Peuter - Mempay - "Hoe kan ik een abonnementsmo...

Lightspeed Connect - Niels de Peuter - Mempay - "Hoe kan ik een abonnementsmo...
Semelhante a Pharmacovigilance Expertise Summary for Startups and Strategic Process Improvement
Semelhante a Pharmacovigilance Expertise Summary for Startups and Strategic Process Improvement (20)
“Pharmaceutical Quality Matrices in determining overall state of Quality and ...

“Pharmaceutical Quality Matrices in determining overall state of Quality and ...
Product Complaints: Complaint Handling from Intake to Closure

Product Complaints: Complaint Handling from Intake to Closure
Transforming Pharmacovigilance from Operational to Scientifically Driven

Transforming Pharmacovigilance from Operational to Scientifically Driven
Computer system validation course pdf september 2017

Computer system validation course pdf september 2017
Effective medical device validation introduction manual advance

Effective medical device validation introduction manual advance
Pharmacovigilance Expertise Summary for Startups and Strategic Process Improvement
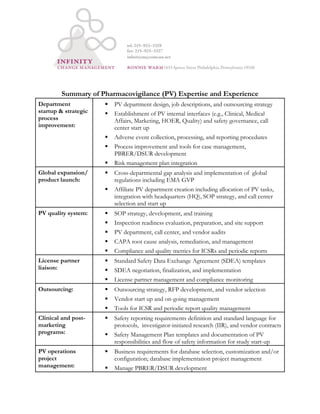
- 1. Summary of Pharmacovigilance (PV) Expertise and Experience Department startup & strategic process improvement: PV department design, job descriptions, and outsourcing strategy Establishment of PV internal interfaces (e.g., Clinical, Medical Affairs, Marketing, HOER, Quality) and safety governance, call center start up Adverse event collection, processing, and reporting procedures Process improvement and tools for case management, PBRER/DSUR development Risk management plan integration Global expansion/ product launch: Cross-departmental gap analysis and implementation of global regulations including EMA GVP Affiliate PV department creation including allocation of PV tasks, integration with headquarters (HQ), SOP strategy, and call center selection and start up PV quality system: SOP strategy, development, and training Inspection readiness evaluation, preparation, and site support PV department, call center, and vendor audits CAPA root cause analysis, remediation, and management Compliance and quality metrics for ICSRs and periodic reports License partner liaison: Standard Safety Data Exchange Agreement (SDEA) templates SDEA negotiation, finalization, and implementation License partner management and compliance monitoring Outsourcing: Outsourcing strategy, RFP development, and vendor selection Vendor start up and on-going management Tools for ICSR and periodic report quality management Clinical and post- marketing programs: Safety reporting requirements definition and standard language for protocols, investigator-initiated research (IIR), and vendor contracts Safety Management Plan templates and documentation of PV responsibilities and flow of safety information for study start-up PV operations project management: Business requirements for database selection, customization and/or configuration; database implementation project management Manage PBRER/DSUR development