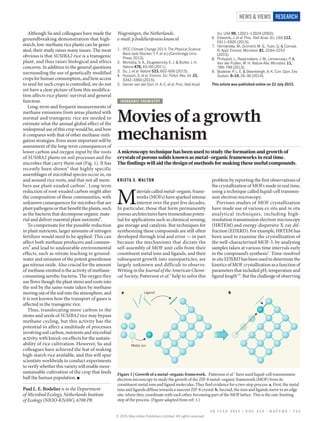
Walton-2015-Nature
- 1. KRISTA S. WALTON M aterials called metal–organic frame- works (MOFs) have sparked intense interest over the past few decades. In particular, those that form permanently porous architectures have tremendous poten- tial for applications such as chemical sensing, gas storage and catalysis. But techniques for synthesizing these compounds are still often developed through trial and error — in part because the mechanisms that dictate the self-assembly of MOF unit cells from their constituent metal ions and ligands, and their subsequent growth into nanoparticles, are largely unknown and difficult to observe. Writing in the Journal of the American Chemi- cal Society, Patterson et al.1 help to solve this problem by reporting the first observations of the crystallization of MOFs made in real time, using a technique called liquid-cell transmis- sion electron microscopy. Previous studies of MOF crystallization have made use of various ex situ and in situ analytical techniques, including high- resolution transmission electron microscopy (HRTEM) and energy-dispersive X-ray dif- fraction (EDXRD). For example, HRTEM has been used to examine the crystallization of the well-characterized MOF-5, by analysing samples taken at various time intervals early in the compound’s synthesis2 . Time-resolved in situ EDXRD has been used to determine the kinetics of MOF crystallization as a function of parameters that included pH, temperature and ligand length3,4 . But the challenge of observing Although Su and colleagues have made the groundbreaking demonstration that high- starch, low-methane rice plants can be gener- ated, their study raises many issues. The most obvious is that SUSIBA2 rice is a transgenic plant, and thus raises biological and ethics concerns. In addition to the general questions surrounding the use of genetically modified cropsforhumanconsumption,andhowaccess to seed for such crops is controlled, we do not yet have a clear picture of how this modifica- tion affects rice plants’ survival and general function. Long-term and frequent measurements of methane emissions from areas planted with normal and transgenic rice are needed to estimate what the annual global effect of the widespread use of this crop would be, and how it compares with that of other methane-miti- gation strategies. Even more important will be assessment of the long-term consequences of lower carbon and oxygen input by the roots of SUSIBA2 plants on soil processes and the microbes that carry them out (Fig. 1). It has recently been shown6 that highly specific assemblages of microbial species occur in, on and around rice roots, and that not all mem- bers use plant-exuded carbon7 . Long-term reduction of root-exuded carbon might alter the composition of these communities, with unknown consequences for microbes that are plant pathogens or that benefit the plants, such as the bacteria that decompose organic mate- rial and deliver essential plant nutrients8 . To compensate for the possible reduction in plant nutrients, larger amounts of nitrogen fertilizer would need to be applied. This can affect both methane producers and consum- ers9 and lead to undesirable environmental effects, such as nitrate leaching to ground water and emission of the potent greenhouse gas nitrous oxide. Also crucial for the amount of methane emitted is the activity of methane- consuming aerobic bacteria. The oxygen they useflowsthoughtheplantstemsandrootsinto the soil by the same route taken by methane movingoutofthesoilintotheatmosphere,and it is not known how the transport of gases is affected in the transgenic rice. Thus, translocating more carbon to the stems and seeds of SUSIBA2 rice may bypass methane cycling, but this activity has the potential to affect a multitude of processes involving soil carbon, nutrients and microbial activity, with knock-on effects for the sustain- ability of rice cultivation. However, Su and colleagues have achieved the feat of making high-starch rice available, and this will spur scientists worldwide to conduct experiments to verify whether this variety will enable more- sustainable cultivation of the crop that feeds half the human population. ■ Paul L. E. Bodelier is in the Department of Microbial Ecology, Netherlands Institute of Ecology (NIOO-KNAW), 6708 PB INORGANIC CHEMISTRY Movies of a growth mechanism A microscopy technique has been used to study the formation and growth of crystals of porous solids known as metal–organic frameworks in real time. The findings will aid the design of methods for making these useful compounds. a b Metal ion Ligand Wageningen, the Netherlands. e-mail: p.bodelier@nioo.knaw.nl 1. IPCC Climate Change 2013: The Physical Science Basis (eds Stocker, T. F. et al.) (Cambridge Univ. Press, 2013). 2. Montzka, S. A., Dlugokencky, E. J. & Butler, J. H. Nature 476, 43–50 (2011). 3. Su, J. et al. Nature 523, 602–606 (2015). 4. Hussain, S. et al. Environ. Sci. Pollut. Res. Int. 22, 3342–3360 (2015). 5. Denier van der Gon, H. A. C. et al. Proc. Natl Acad. Figure 1 | Growth of a metal–organic framework. Patterson et al.1 have used liquid-cell transmission electron microscopy to study the growth of the ZIF-8 metal–organic framework (MOF) from its constituent metal ions and ligand molecules. They find evidence for a two-step process. a, First, the metal ions and ligands diffuse towards a nascent ZIF-8 crystal. b, Second, the ions and ligands move to an edge site, where they coordinate with each other, becoming part of the MOF lattice. This is the rate-limiting step of the process. (Figure adapted from ref. 1.) Sci. USA 99, 12021–12024 (2002). 6. Edwards, J. et al. Proc. Natl Acad. Sci. USA 112, E911–E920 (2015). 7. Hernández, M., Dumont, M. G., Yuan, Q. & Conrad, R. Appl. Environ. Microbiol. 81, 2244–2253 (2015). 8. Philippot, L., Raaijmakers, J. M., Lemanceau, P. & Van der Putten, W. H. Nature Rev. Microbiol. 11, 789–799 (2013). 9. Bodelier, P. L. E. & Steenbergh, A. K. Curr. Opin. Env. Sustain. 9–10, 26–36 (2014). This article was published online on 22 July 2015. 3 0 J U L Y 2 0 1 5 | V O L 5 2 3 | N A T U R E | 5 3 5 NEWS & VIEWS RESEARCH © 2015 Macmillan Publishers Limited. All rights reserved
- 2. the crystallization process in real time persists. Patterson and colleagues’ use of liquid-cell transmission electron microscopy (LCTEM) is a big step forward. This technique allows dynamic processes that occur in liquids to be imaged as they happen. It has been used to observe systems such as biological structures5 and growing nanocrystals6 , but had not previ- ously been applied to MOF syntheses. Because analytical samples can be damaged by the electron beam used in LCTEM, the authors began by performing a series of con- trol experiments using a zirconium-based MOF called UiO-66, to decouple the effects of beam irradiation on MOF synthesis from the effects of the reaction mechanism. UiO-66 was a good choice for a control because it is easy to synthesize and extremely stable, which meant that it could be prepared ahead of the LCTEM experiments without any risk of it degrading before use. The authors observed that the dissolution or growth of UiO-66 par- ticles depends on the voltage of the electron beam. A threshold dosage of 40,000 electrons per square nanometre was also established — as long as experiments were performed below this limit, damage and particle motion during crystallization were negligible. Patterson et al. then chose another MOF, ZIF-8, as the ideal candidate for demonstrat- ing the LCTEM method. The growth mecha- nisms of ZIF-8 have previously been studied7 using transmission electron microscopy on samples removed from synthetic solutions of the MOF, which provided a good comparison with the LCTEM results. ZIF-8 can be synthe- sized at room temperature in methanol, using zinc nitrate as the metal source and 2-methyl imidazole molecules as the organic ligands. The authors observed the growth of ZIF-8 in real time over 11 minutes — the first particles detected were 15 nm in diameter, with subse- quent growth observed up to 50 nm. The authors went on to record videos of the particleformationandusedthemtodetermine the growth kinetics of the MOF, uncovering several important features of the crystalliza- tion process. First, they proved by direct obser- vation that ZIF-8 particles form through the growth of smaller subunits, rather than by particles coalescing. They also found that an excess of ligand molecules leads to the forma- tion of ZIF-8 particles that are smaller than those formed when the metal-to-ligand ratio is 1:1. The researchers had predicted this using ex situ methods, but LCTEM enabled them to observe the process as it occurred. A series of careful growth experiments was then performed under various accumulative electron doses. The results convincingly show that LCTEM can be applied effectively to study nanoparticles that are easily damaged by elec- tron beams. It remains to be seen whether the technique will be effective at the higher tem- peratures (typically greater than 100 °C) at which most MOFs form. A general conclusion from this work is that MOF growth occurs through the transport of metals and ligands to a nascent particle, fol- lowed by their movement to an edge or surface site, where bonding between the metal and ligand finally occurs (Fig. 1). The attachment of metal–ligand monomers to a surface site is therefore the controlling factor in particle growth,andtheprocessisnotdiffusion-limited. The development of this ability to watch particle formation in situ during MOF self- assembly should enable a variety of compli- cated synthetic questions to be answered. One example is how the addition of ‘modulator’ compounds, which are sometimes used in MOF syntheses to control the crystallinity of the products, affects the growth kinetics. For MOFs that can adopt different structures, LCTEM could also shed light on what dictates whether kinetic products — those that crys- tallize most quickly — form during reactions, ratherthanthemostthermodynamicallystable products. LCTEM is a much-needed addition to the MOF-characterization toolkit, and its use in conjunction with other methods will no doubt lead to the specific control of crystal morphology, compositions and defects. ■ Krista S. Walton is at the School of Chemical and Biomolecular Engineering, Georgia Institute of Technology, Atlanta, Georgia 30332, USA. e-mail: krista.walton@chbe.gatech.edu 1. Patterson, J. P. et al. J. Am. Chem. Soc. 137, 7322–7328 (2015). 2. Zheng, C., Greer, H. F., Chianga, C.-Y. & Zhou, W. CrystEngComm 16, 1064–1070 (2014). 3. Ragon, F., Chevreau, H., Devic, T., Serre, C. & Horcajada, P. Chemistry 21, 7135–7143 (2015). 4. Ahnfeldt, T. et al. Chemistry 17, 6462–6468 (2011). 5. de Jonge, N., Peckys, D. B., Kremers, G. J. & Piston, D. W. Proc. Natl Acad. Sci. USA 106, 2159–2164 (2009). 6. Liao, H.-G., Niu, K. & Zheng, H. Chem. Commun. 49, 11720–11727 (2013). 7. Venna, S. R., Jasinski, J. B. & Carreon, M. A. J. Am. Chem. Soc. 132, 18030−18033 (2010). MATERIALS SCIENCE Composite for energy storage takes the heat A polymer-based material has been discovered that breaks the rules — it has the right combination of properties for use in energy-storage devices called dielectric capacitors, and can function at high temperatures. See Letter p.576 HARRY J. PLOEHN D evices known as dielectric capacitors have a crucial role in applications that require short, intense power pulses or the conversion of direct current to alternating current. These applications include electronic systems for the integration of energy from renewable sources into power grids1 , trans- port2 and military weapon systems3 . They depend on electrically insulating materials known as dielectrics, which come in several types. Polymeric dielectrics offer advantages forlargecapacitors,butsufferfromlowoperat- ing temperatures (usually well below 150 °C) and low energy density (which means that devices that use polymeric dielectrics occupy large volumes). On page 576 of this issue, Li et al.4 report that a composite of a polymer and nanometre-scale sheets of boron nitride pro- vides more than a 40% improvement in energy density compared with the best-available poly- mer dielectric, as well as remarkable stability at temperatures up to 300 °C across a wide range of electric-field frequencies. Dielectric capacitors achieve the highest rate of energy transfer (termed the power or rate capability) of all capacitor types. They store energy through a variety of molecular and nanoscale electron-polarization mecha- nisms5,6 that create oriented dipoles and associated dipolar electric fields. For high energy density, dielectric materials must have a high density of dipoles that have large induced dipole moments (which provide a measure of a charged system’s polarity). A dielectric’s rate capability depends on how fast charges polarize and depolarize — how fast the dipoles reorient — as an applied elec- tric field varies. Invariably, not all of the energy stored in dipolar electric fields is recovered on depolarization; some is transferred into molecular translation and vibration (thermal energy) and is lost as heat, a process called dielectric loss. When and how polarized electrons begin to ‘leak’ (conduct) through a dielectric depends on a property called the dielectric breakdown field strength (Eb). Relatively small leakage currents may occur at field strengths below Eb. Once the field reaches Eb, it promotes a cas- cadeofelectronsintothematerial’sconduction band, resulting in catastrophic breakdown as the dielectric is transformed from an insulator 5 3 6 | N A T U R E | V O L 5 2 3 | 3 0 J U L Y 2 0 1 5 NEWS & VIEWSRESEARCH © 2015 Macmillan Publishers Limited. All rights reserved