Chemistry-Introduction_150921_01e
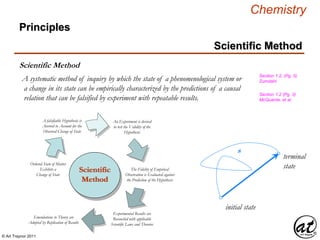
- 1. © Art Traynor 2011 Chemistry Principles Scientific Method Section 1.2, (Pg. 5) Zumdahl ? ? ? ? Pro-Forma (Secret Shopper) Audit/Survey Pro-Forma (Secret Shopper) Audit/Survey Overture Letter/Proposal Overture Letter/Proposal Permissive Store Visit or Mockup Permissive Store Visit or Mockup Scientific Method ? Ordered State of Matter Exhibits a Change of State The Fidelity of Empirical Observation is Evaluated against the Prediction of the Hypothesis Emendations to Theory are Adopted by Replication of Results s initial state terminal state A falsifiable Hypothesis is Averred to Account for the Observed Change of State An Experiment is devised to test the Validity of the Hypothesis Scientific Method A systematic method of inquiry by which the state of a phenomenological system or a change in its state can be empirically characterized by the predictions of a causal relation that can be falsified by experiment with repeatable results. Experimental Results are Reconciled with applicable Scientific Laws and Theories Section 1.2 (Pg. 3) McQuarrie, et al.
- 2. © Art Traynor 2011 Chemistry Principles Scientific Method Scientific Method A methodology for characterizing the behavior of a phenomenological system. The system must admit measurement Section 1.2, (Pg. 4) Phenomena Hypothesis Empirical ( Measurable ) Theory ( Conjecture ) Falsifiable ( Refutable ) Replicable ( Reproducible ) Systematic Experiment Observation Organized Predictable Qualitative Quantitative Procedure ( Operation) Measurement Number Unit Testing Interpretive Results Model Explanative Refining Consistent System Process ( Reaction ) Behavior Logical Consequence Population Null – Alternative Formulation Analysis Question Simplicity Principle of Parsimony Occam’s Razor Correlation Causation Conflation Selection Bias Confirmation Bias Hindsight Bias (Postdiction) Control Accuracy Precision Uncertainty ( Error ) Confirmation Heuristic Data
- 3. © Art Traynor 2011 Chemistry Principles Phenomenological System Phenomenological System An ordered state of matter possessed of attributes capable of being characterized by a descriptive quantification Section 1.2, (Pg. 5) Zumdahl
- 4. © Art Traynor 2011 Chemistry Principles Scientific Law Law of Science It’s not so much a Law… as a Good Idea A durable declaration – premised on uncontroverted, aggregated observation, such as to suggest universal validity – inferring the operation of a causal relation accounting for a particular change of state within a phenomenological system. A Scientific Law should not be thought of a Theory that has graduated status with the accumulation of additional evidence Section 1.2, (Pg. 5) Zumdahl Section 1.2 (Pg. 3) McQuarrie, et al. Does not proffer Explanation ! Summarizes a Relationship
- 5. © Art Traynor 2011 Chemistry Principles Scientific Theory A Theory is typically much broader in scope than a Scientific Law, which is much more limited in the scope of its explanatory compass Section 1.2, (Pg. 5)Theory A well-substantiated explanation – premised on systematic observation and experiment ( typical of The Scientific Method ) – inferring the existence of a causal relation accounting for a particular change of state within a phenomenological system. A valid theory is characterized by the following: Falsifiable Prediction with Consistent Accuracy Empirically Premised on Observation and/or Experiment Parsimonious as to Alternatives Adaptive to Supplemental Data Replicable Results Explanation explanandum less obvious more obvious elucidation A Theory can incorporate several Scientific Laws Represents a Unification of Ideas ( derivative of one or more laws ) Cannot be Proven Correct
- 6. © Art Traynor 2011 Chemistry Principles Scientific Theory Section 1.2, (Pg. 5)Theory A well-substantiated explanation – premised on systematic observation and experiment ( typical of The Scientific Method ) – inferring the existence of a causal relation accounting for a particular change of state within a phenomenological system. Logical Positivistic Explanation explanandum less obvious more obvious elucidationNumerous epistemological interpretations of the Scientific Method have emerged to enrichen its vitality: Theories are to be considered as analogous to mathematical axioms – a propositional form
- 7. © Art Traynor 2011 Chemistry Principles Scientific Theory Section 1.2, (Pg. 5)Theory A well-substantiated explanation – premised on systematic observation and experiment ( typical of The Scientific Method ) – inferring the existence of a causal relation accounting for a particular change of state within a phenomenological system. Explanation explanandum less obvious more obvious elucidationNumerous epistemological interpretations of the Scientific Method have emerged to enrichen its vitality: Models Theories are to be considered as analogous to mathematical axioms
- 8. © Art Traynor 2011 Chemistry Principles Hypothesis Hypothesis A conjecture arising from the formulation of an empirical (scientific) question which supplies a plausible explanation to account for a particular change of state observed to occur within a phenomenological system. Null Hypothesis Alternative Hypothesis The conjecture that experiment will falsify a relation posited to account for a particular change of state observed to occur within a phenomenological system. The preferred conjecture that experiment will verify a relation posited to account for a particular change of state observed to occur within a phenomenological system. Essentially synonymous with Conjecture, however Conjecture is more formally defined in Mathematics as a Proposition for which a Proof has yet to be stated
- 9. © Art Traynor 2011 Chemistry Principles Hypothesis Hypothesis A conjecture arising from the formulation of an empirical (scientific) question which supplies a plausible explanation to account for a particular change of state observed to occur within a phenomenological system. A distillation of Question Formulation Criteria as proposed by Dietrich Dörner with subsequent expansion /refinement by Joachim Funke Transparency ( Intransparency ) Polytely ( Objective Multiplicity/Profusion , “ mission creep ” ) Complexity Dynamics
- 10. © Art Traynor 2011 Chemistry Principles Experiment Experiment An ordered procedure by which to establish the validity of a Hypothesis purporting to characterize a phenomenological system or to otherwise account for any observed change of state within the system. Observation Qualitative – phenomena are characterized according to Class Equivalencies Color Odor Reactivity Quantitative – susceptible to measurement Section 1.2 (Pg. 5) McQuarrie, et al.
- 11. © Art Traynor 2011 Chemistry Principles Chemistry Chemistry A discipline within the Physical Sciences dedicated to the explication of the Composition Structure Properties Changes of State Of ( or within ) a phenomenological system. Chemistry seeks to describe the properties of Individual Atoms ( Elements ) Bonding Intermolecular Forces Reactions Chemistry is regarded as a “ Central Science ” as its precepts are of equal explanatory force within the related disciplines of Physics, Geology, and Biology Wiki “Chemistry”
- 12. © Art Traynor 2011 Chemistry Principles Chemistry Chemistry The etymology of “ Chemistry ” can be traced through several alternatively plausible origins Alchemy al-kīmīā ( Arabic ) χημεία or chemeia ( Greek – “ cast together ” ) Khem – ( ancient Greek name for Egypt, for their prowess in metalworking ) Wiki “Chemistry” Kim Mi – ( Chinese for “ the secret of Gold ” )
- 13. © Art Traynor 2011 Chemistry Principles Measurement Measurement A quantity ascertained by observation characterized by: Magnitude Unit The Modulus or Absolute Value, | m | assigned to represent the observation. The ordering Metric by which a Relation with the magnitude of the observation might be expressed as a scaled ratio. Section 1.3, (Pg. 7) Uncertainty A parameter associated with an observation relating the probabilistic dispersion of the Magnitude. Measurand – that quantifiable aspect of a phenomenological system by which class equivalences can be metrically determined Measurand – the object of the measurement Measurement – the estimation of the ratio (typically attribute differences) between the magnitude of a continuous quantity and a unit magnitude of the same class.
- 14. © Art Traynor 2011 Chemistry Principles Measurement – Qualities of Unit Quantities Unit quantities can be classed as one of two types: Extensive Quantities Classes of phenomena admitting a Concatenation operation . Section 1.3, (Pg. 7) Also known as Base Quantities Examples: Length, Mass, Time, Electrical Resistance, Plane Angle, etc. Intensive Quantities Classes of phenomena not admitting a Concatenation operation . Examples: Temperature, etc. Measurement
- 15. © Art Traynor 2011 Chemistry Measurement SI Units Systèm Internationale ( SI ) Fundamental SI Units Mass Length Time Temperature Electric Current Substance Cardinality Luminous Intensity kilogram meter second Kelvin ampere mole candela kg m s K A mol cd There are only seven Fundamental SI Units “Dimension” is rigorously defined as the indexed product of a fundamental physical Metric ( of which there are precisely five ) Dimension Name Abbreviation Section 1.4, ( Pg. 11 ) Variable m l t T I n The notion of “Dimension” is hierarchically distinct from the notion of a “ Scale Unit ” “Dimension” is conceived to describe a distinct phenomenological equivalence class on which a relation to a measurand can be defined by a scaled ratio A “Fundamental” unit is one from which every other measure in its phenomenological class can be derived (akin to vector Basis, or Linearly Independent Set)
- 16. © Art Traynor 2011 Chemistry Measurement SI Units Systèm Internationale ( SI ) Additional SI (?) Units Physical Quantity Name Abbreviation Variable Volume Liter L v Inductance Henry H L Units which can be “ factored ” into other units, or are com- positions of one or more Base Units are not fundamental, ( e.g. a volume in Liters can be equally stated in terms of length as in 1mL = 1cm3 , or velocity expressed in mi/hr ) Capacitance Farad F C Resistance Ohm Ω R Electro-Motive Force Volt V E Electric Charge Coulomb C Q
- 17. © Art Traynor 2011 Chemistry Measurement SI Units Systèm Internationale ( SI ) Additional Units – Compound Units Physical Quantity Name Abbreviation Variable Density Kilogram L Compound Units A composition of measures drawn from two or more of the fundamental units. Liter kg ⍴= kg · L – 1 v m =
- 18. © Art Traynor 2011 Chemistry Measurement SI Units Systèm Internationale ( SI ) Fundamental SI Units Mass A measure characterizing the Inertia of a body, or its resistance to a change in its state of motion ( i.e. the Force necessary to impart a certain acceleration ). Weight The response of a Mass to the Force of Gravity . Temperature A measure of the relative tendency of heat to escape a radiant body . Section 1.2 (Pg. 5) McQuarrie, et al.
- 19. © Art Traynor 2011 Chemistry Measurement SI Units 10 – 24 10 – 21 10 – 18 10 – 15 10 – 12 10 – 9 10 – 6 10 – 3 10 – 2 10 – 1 Yocto y 10 24YottoY Zepto z 10 21ZettaZ Atto a 10 18ExaE Fempto f 10 15PetaP Pico P 10 12TeraT Nano n 10 9GigaG Micro μ 10 6MegaM Milli m 10 3Kilok Centi c 10 2 Deci d 10 1Dekada Hectoh Systèm Internationale ( SI ) Unit Prefixes
- 20. © Art Traynor 2011 Chemistry Measurement SI Units Systèm Internationale ( SI ) Additional SI Units 1m = 10dm 10dm = 1m 1m = 10dm 1 meter = 10 decimeters Length Volume 1 m3 = 1,000 dm3 or 1,000 L 1dm3 = 1L 1cm3 = 1mL 1 decimeter = 10 centimeters 1 dm3 = 1,000 cm3 or 1 mL 1dm = 10 cm 1dm = 10 cm 1dm = 10 cm
- 21. © Art Traynor 2011 Chemistry Measurement Uncertainty Measurement A quantity ascertained by observation characterized by: Uncertainty A parameter associated with an observation relating the probabilistic dispersion of the Magnitude. As previously noted (essential elements of a Measurement ) Etiology of Error Precision & Accuracy Significant Figures ( Sig Figs )
- 22. © Art Traynor 2011 Chemistry Measurement Error Measurement A quantity ascertained by observation characterized by: Uncertainty A parameter associated with an observation relating the probabilistic dispersion of the Magnitude. As previously noted (essential elements of a Measurement ) Etiology of Error n Systematic Error Error which is introduced into a measurement by a defect in the measurement device n Sensitivity Error Error which is introduced into a measurement by an overspecification of device precision Characterized by a posteriori “ predictable ” error
- 23. © Art Traynor 2011 Chemistry Measurement Measurement A quantity ascertained by observation characterized by: Uncertainty A parameter associated with an observation relating the probabilistic dispersion of the Magnitude. As previously noted (essential elements of a Measurement ) Etiology of Error n Random Error Non-systematic error ( typically ) introduced into the measurement by factors other than those inhering to the measurement device ( e.g. human factors ) Characterized by a posteriori “ unpredictable ” error This species of error can be systematically characterized by a Probability Density Function ( PDF ) o Error
- 24. © Art Traynor 2011 Chemistry The discrepancy between an exact value and some approximation ( measurement ) of it Measurement Measurement A quantity ascertained by observation characterized by: Uncertainty Error Etiology of Error n Approximation Error Swok, Section 11.5, Pg. 560o If “ E” represents error in an approximation, the approximation can be considered accurate to “ k” decimal places if the modulus of “ E” is less than the product of five-tenths and ten indexed to the minus “ k” 1-decimal place Accuracy | E | < 0.5 x 10 – 1 = 0.0.5 ① Moving 1 position in the “+” direction k-decimal place Accuracy | E | < 0.5 x 10 – k 2-decimal place Accuracy | E | < 0.5 x 10 – 2 = 0.0.0.5 Moving 2 positions in the “+” direction ①② 3-decimal place Accuracy | E | < 0.5 x 10 – 3 = 0.0.0.0.5 Moving 3 positions in the “+” direction ①②③
- 25. © Art Traynor 2011 Chemistry The discrepancy between an exact value and some approximation ( measurement ) of it The magnitude of the difference between the exact value and the approximation ( e.g. ± 0.05m ) Magnitudes are always expressed as absolute values and are thus always positive numbers Measurement Measurement A quantity ascertained by observation characterized by: Uncertainty Error Etiology of Error n Approximation Error n Absolute Error ( Tolerance )
- 26. © Art Traynor 2011 Chemistry Measurement Measurement A quantity ascertained by observation characterized by: Uncertainty Error Etiology of Error n Relative / Fractional / Percentage Error The absolute error expressed as a ratio of the exact value ( e.g. 56.47 ± 0.02mm ) 0.02mm 56.47mm = Absolute Error Exact Value = 0.0004 → ( 0.0.0.04 ) ( 100% ) = 0.04% Relative Error ➀ ➁ Percentage Error
- 27. © Art Traynor 2011 Chemistry Measurement Examples: 843.6m or 843.0m or 800.0m implies a margin of error of 0.05m or ± 0.05m 843.55m ≤ x ≤ 843.65m (nominal 843.6m) 842.95m ≤ x ≤ 843.05m (nominal 843.0m) 800.95m ≤ x ≤ 800.05m (nominal 840.0m) An interval composed such that its mean is situated equidistant to a difference and sum of the absolute error Measurement A quantity ascertained by observation characterized by: Uncertainty Error Etiology of Error n Margin of Error
- 28. © Art Traynor 2011 Chemistry Measurement Precision & Accuracy Measurement A quantity ascertained by observation characterized by: Uncertainty A parameter associated with an observation relating the probabilistic dispersion of the Magnitude. As previously noted (essential elements of a Measurement ) Precision & Accuracy Instruments of measurement feature a graduated scale ( enumerated intervals incremented in the ordering metric appropriate to the measurand aspect to be quantified/recorded, e.g. weight, volume, length ). 1 2 3 mm Measurement Instrument Precision ( MIP ) A measurement instrument’s precision is given by the magnitude of its least graduation Measurement Reading Precision ( MRP ) The measure to be recorded will be 1/10 the MIP MIP 10 = = 0.001 x 10 – 1 MIP = 0.001M = 1mm MRP = 0.0.001 M = 0.1mm ➀ 0.001M 10 MIP MRP n n
- 29. © Art Traynor 2011 Chemistry Measurement Precision & Accuracy Measurement A quantity ascertained by observation characterized by: Uncertainty Precision & Accuracy Certain Digits ( CD ) The digital cardinality ( i.e. count of digits ) of the MIP constitute that part of the measurement which is certain | MIP | = n = CD Uncertain Digits ( UCD ) The additional digit supplied by the MRP is estimated and thus considered Uncertain | MRP | = n + 1 = UCD Significant Figures of Measurement ( SFOM ) The count of all certain digits plus that of the first uncertain digit n n n
- 30. © Art Traynor 2011 Chemistry Precision The degree of closeness to which a quantitative measurement approximates the true value of the quantity measured Measurement Precision & Accuracy The agreement of a particular experimental result with its true result Section 1.4, ( Pg. 11 ) Measurement A quantity ascertained by observation characterized by: Uncertainty Precision & Accuracy Random or Indeterminate Error Introduces an equal probability of the measurement registering either high or low from its true value Ideal Accurate & Precise Neither Accurate nor Precise n o
- 31. © Art Traynor 2011 Chemistry Accuracy The degree to which repeated measurements (under unchanged conditions) yield the same results Measurement Precision & Accuracy The agreement of a particular experimental result with its true result Section 1.4, ( Pg. 11 ) Measurement A quantity ascertained by observation characterized by: Uncertainty Precision & Accuracy Systematic or Determinate Error Introduces a bias error manifesting in a consistent deviation in experimental result from the measurand true value Ideal Accurate & Precise Precise but not Accurate n o
- 32. © Art Traynor 2011 Chemistry Measurement Precision & Accuracy Measurement A quantity ascertained by observation characterized by: Significant Figures ( Sig Figs ) Significant Figures of Measurement ( SFOM ) Uncertainty Significant Figures of Computation ( SFOC ) Exponential ( Scientific ) Notation Normalized Scientific Notation ( NSN ) Engineering Scientific Notation ( ESN ) n n n n n
- 33. © Art Traynor 2011 Chemistry Measurement Precision & Accuracy Measurement A quantity ascertained by observation characterized by: Significant Figures ( Sig Figs ) Significant Figures of Measurement ( SFOM ) The count of all certain digits plus that of the first uncertain digit Uncertainty As previously noted (essential elements of a Measurement ) Significant Figures of Computation ( SFOC ) All non-zero digits are considered significant Zeros bounded by non-zeros are significant Leading zeros are not significant Trailing zeros following a decimal point are significant Trailing zeros not accompanied by a decimal point are ambiguous A decimal point may be placed after the number to ratify the significance of the trailing zeros Integers or Fractions are considered to be significant “ Captive ” Zeros Section 1.4, ( Pg. 13 ) “ Leading ” Zeros do nothing more than locate a (string of) sig figs relative to a decimal numerical representation n n o o o o o o
- 34. © Art Traynor 2011 Chemistry Measurement Precision & Accuracy Measurement A quantity ascertained by observation characterized by: Significant Figures ( Sig Figs ) Significant Figures of Measurement ( SFOM ) The count of all certain digits plus that of the first uncertain digit Uncertainty As previously noted (essential elements of a Measurement ) Significant Figures of Computation ( SFOC ) Exact Numbers are considered significant Sect 1.5, pg 9 Section 1.4, ( Pg. 13 ) “ Exact ” Numbers are those not derived from measurement, akin to discrete enumeration derived from counting, or Ex Vi Termini ( EVT – from definition ), which have an unlimited number of Sig Figs Exponential ( Scientific ) Notation whereby a number with a surfeit of zeros (either large or small in relative magnitude) or otherwise populated by digits beyond those necessary for the desired precision ( sig figs ) A species of mathematic operation (exponentiation) is alternatively expressed as the product of a coefficient ( reduced to only its sig figs ) and a multiplier-constant (ten) indexed by an integer. a x 10b n n n o
- 35. © Art Traynor 2011 Chemistry Measurement Precision & Accuracy Measurement A quantity ascertained by observation characterized by: Significant Figures ( Sig Figs ) Uncertainty Exponential ( Scientific ) Notation whereby a number with a surfeit of zeros (either large or small in relative magnitude) or otherwise populated by digits beyond those necessary for the desired precision ( sig figs ) A species of mathematic operation (exponentiation) is alternatively expressed as the product of a coefficient ( reduced to only its sig figs ) and a multiplier-constant (ten) indexed by an integer. a x 10b Example: “ 350 ” 350 = 3.5.0.0 = 3.5 x 102 Representing integers by scientific notation (resultant) entails movement of the decimal in the “ + “ direction ① ② Moving 2 positions in the “+” direction 350 = 35.0.0 = 35.0 x 101 ① Moving 1 position in the “+” direction 350 = 350.0 = 350.0 x 100 i Moving 0 positions in the “+” direction n
- 36. © Art Traynor 2011 Chemistry Measurement Precision & Accuracy Measurement A quantity ascertained by observation characterized by: Significant Figures ( Sig Figs ) Uncertainty Exponential ( Scientific ) Notation a x 10b Normalized Scientific Notation ( NSN ) “ a ” is bounded between one and ten, 1 ≤ |a | < 10 , which allows for easy comparison of two numbers so expressed as the exponent “ b ” in this form represents the product’s order of magnitude The exponent “ b ” is chosen so that the absolute value of the coefficient For numbers with absolute value between zero and one, 0 < |a | < 1 the exponent b, is expressed as a negative index (e.g. – 5 x 10-1 ) Examples: – 0.5 = – 0.5.0 = – 5.0 x 10-1 ➀ Moving 1 position in the “–” direction Representing a decimal by scientific notation (resultant) entails movement of the decimal in the “ – “ direction Wikipedia n n o
- 37. © Art Traynor 2011 Chemistry Measurement Precision & Accuracy Measurement A quantity ascertained by observation characterized by: Significant Figures ( Sig Figs ) Uncertainty In ESN the exponent “ b ” is restricted to multiples of three so that the absolute value of the coefficient “ a ” lies between one and one-thousand, 1 ≤ |a | < 1000 , which allows for easy comparison of two numbers so expressed as the exponent “ b ” corresponds to specific SI prefixes Example: “ 0.0000000125m ” → 12.5 x 10-9m “ twelve-point-five nanometers ” 1.25 x 10-8m “ one-point-two-five times ten-to-the-negative-eight meters ” ⑨ ( 0.0.0.0.0.0.0.0.1.25 ) = 1.25 x 10-8 m ⑧① ②③ ④ ⑤⑥ ⑦ Moving 8 positions in the “–” direction ( 0.0.0.0.0.0.0.0.1.2.5 ) = 12.5 x 10-9 m ⑧① ②③ ④ ⑤⑥ ⑦ Moving 9 positions in the “–” direction Engineering Scientific Notation ( ESN ) o Wikipedian o
- 38. © Art Traynor 2011 Chemistry Measurement Precision & Accuracy Measurement A quantity ascertained by observation characterized by: Significant Figures ( Sig Figs ) Uncertainty Significant Figures of Computation ( SFOC ) o Arithmetic: Addition/Subtraction The number of significant figure decimal places in the sum or difference of the operation should equal the smallest number of decimal places in any of the operands Multiplicative: Multiplication/Division The number of significant figures in the product or quotient of the operation is the same as the number of significant figures in the least accurate of the operands (having the lowest number of significant figures) n o
- 39. © Art Traynor 2011 Chemistry Measurement Precision & Accuracy Measurement A quantity ascertained by observation characterized by: Significant Figures ( Sig Figs ) Uncertainty Significant Figures of Computation ( SFOC ) o Arithmetic: Addition/Subtraction The number of significant figure decimal places in the sum or difference of the operation should equal the smallest number of decimal places in any of the operands Example: 123 + 5.35 ≠ 128.35 = 128 3 Sig Figs, 0 Decimal Places 3 Sig Figs, 2 Decimal Places 5 Sig Figs > 3 Sig Figs (2 Decimals) 3 Sig Figs, 0 Decimals n
- 40. © Art Traynor 2011 Chemistry Measurement Precision & Accuracy Measurement A quantity ascertained by observation characterized by: Significant Figures ( Sig Figs ) Uncertainty Significant Figures of Computation ( SFOC ) o Multiplicative: Multiplication/Division L WA = l x w Example: L : 16.3cm ± 0.1cm { 16.2cm – 16.4cm } W : 4.5cm ± 0.1cm { 4.4cm – 4.6cm } A : 16.3cm x 4.5cm ≠ 73.35cm 2 { 71cm – 75cm } = 73cm 2 3 Sig Figs 2 Sig Figs 4 Sig Figs > 2 Sig Figs 2 Sig Figs Serway, pg 15 n
- 41. © Art Traynor 2011 Chemistry Measurement Precision & Accuracy Measurement A quantity ascertained by observation characterized by: Significant Figures ( Sig Figs ) Uncertainty Significant Figures of Computation ( SFOC ) o Rounding: Round to Integer ( RTI ) There is at least one (1) non-direct method to produce round-to-integer results Round To Nearest – “ q” is the integer that is closest to “ y” “ y” is the number to be rounded ( y ℝ ) “ q” is the integer result ( q ℤ ) of the rounding operation n
- 42. © Art Traynor 2011 Chemistry Measurement Precision & Accuracy Measurement A quantity ascertained by observation characterized by: Significant Figures ( Sig Figs ) Uncertainty Significant Figures of Computation ( SFOC ) o Rounding: Round to Integer ( RTI ) There is at least one (1) non-direct method to produce round-to-integer results Tie-Breaking Rule for when “ y” is half-way between two integers, i.e. y = 0.5 Round Half-Up or round half towards +∞ q = : ⌊ y + 0.5⌋ = – ⌈ – y – 0.5⌉ Examples: ⌊ 23.5 + 0.5⌋ = 24 ; – ⌈ – ( – 23.5 ) – 0.5⌉ = – 23 Rosen, pg 149 n
- 43. © Art Traynor 2011 Chemistry Dimensional Analysis Measurement – Qualities of Unit Quantities Section 1.6, (Pg. 17) Unit Factors Insofar as a principal salient of a phenomenological system is its ability to be characterized by a descriptive quantification, a well formed quantification ( i .e. measurement ) thereby consists of the following: An ordered state of matter possessed of attributes capable of being characterized by a descriptive quantification Phenomenological System Unit of Measure ( UOM ) A fundamental or ordering Metric, characteristic of the measurand and by which class equivalencies with like phenomena can be gauged, by which a Relation with the magnitude of an observation might be expressed as a scaled ratio. Magnitude The Modulus or Absolute Value, | m | assigned to represent an observation and by which a scaled ratio relation to the UOM can be made . Examples: Length: 23.4 km ( Kilometers / Meters ) Mass: 38.5 lbs ( Pounds ) Time: 1:32 hrs ( Hour : Seconds ) Current: 0.32 mA ( Miliamps / Amperes ) | m | UOM
- 44. © Art Traynor 2011 Chemistry Dimensional Analysis Measurement – Qualities of Unit Quantities Section 1.6, (Pg. 17) Unit Factors Unit of Measure ( UOM ) Magnitude Fundamental or Base Unit A fundamental or ordering Metric, characteristic of the measurand and by which class equivalencies with like phenomena can be gauged, by which a Relation with the magnitude of an observation might be expressed as a scaled ratio. A UOM from which every other measure in its phenomenological class can be derived. Akin to a “Basis” or Linearly Independent Set Mass Length Time Temperature Electric Current Substance Cardinality Luminous Intensity kilogram meter second Kelvin ampere mole candela kg m s K A mol cd The Seven Fundamental SI Units Units which can be “ factored ” into other units, or are compositions of one or more Base Units are not fundamental, ( e.g. a volume in Liters can equally stated in terms of length as in be expressed as 1mL = 1cm3 , or velocity expressed in mi/hr ) n n
- 45. © Art Traynor 2011 Chemistry Dimensional Analysis Measurement – Qualities of Unit Quantities Section 1.6, (Pg. 17) Unit Factors Unit of Measure ( UOM ) Magnitude Fundamental or Base Unit Conversion Factor ( CF ) A ratio ( constituted of disparate UOMs evaluating to a unity ) which has the multiplicative effect of converting one UOM into another without affecting the magnitude of the quantity as between the operands and ( resultant ) product. Dimensional Invariance The operation of a physical law will hold proportionally irrespective of the UOM employed by which to assay its effects (e.g. two buildings will measure the same height whether measured in feet or meters).
- 46. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Unit Factors Method of Dimensional Analysis ( MODA ) Dimensional Homogeneity Only commensurable quantities ( those with identical dimension) may be ( algebraically ) manipulated to permit: The veracity of a computational result can frequently be gauged by the agreement of the solution UOMs with that of those expected to be ascertained. Dimensional Fidelity Comparison Equality Addition Difference Incommensurable quantities, expressed as a ratio however, may be employed as Conversion Factors ( CF’s ) indirectly enabling the relations of commensurable quantities to be evaluated. Quantities expressed in formulae are said to be either “ Like Dimensioned ” (in which case operations of their Abelian Group can be directly performed) or “ Unlike Dimensioned ” in which case those operations might still be performed if those UD quantities are expressed as ratios
- 47. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Unit Factors Method of Dimensional Analysis ( MODA ) Factor Label Method ( FLM ) or Unit Factor Method ( UFM ) Problem Solving Technique UOMi 1 ① UOMf 1 O’Leary, Section 8.3, Pg. 346 Akin to a Cantor Set forming iteration UOMi 1 → State both the Initial and Final terms as rationals of unity indicating the progression of the calculation LHS → RHS An equation is constructed, with the Initial term occupying the LHS and the Final term occupying the RHS of the equality. At least one CF will be necessary ( presuming a CF rational featuring UOMf units as dividend and with UOMi units for a divisor can be furnished for the calculation) in which case the Final term is replicated on the RHS as the singular CF = CFf UOMf 1 UOMf 1 = Initial FinalFinal Initial CFf Final UOMi 2a
- 48. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Unit Factors Method of Dimensional Analysis ( MODA ) Factor Label Method ( FLM ) or Unit Factor Method ( UFM ) Problem Solving Technique UOMi 1 Should a single CF rational with UOMf numerator and UOMi unit denominator not be available for the calculation, at least one additional CF will need to be introduced. UOMf 1 UOMf 1 = Initial CFf Final CF’s necessary to complete the transformation will be inserted here UOMi 1 Lacking for a singular CF, the additional CF , denoted CFk , will be interpolated, and juxtpositioned between the Initial term and CFf UOMi 1 UOMf 1 UOMf 1 Initial CFk CFf Final ③ = 2b
- 49. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Unit Factors Method of Dimensional Analysis ( MODA ) Factor Label Method ( FLM ) or Unit Factor Method ( UFM ) Problem Solving Technique The interpolated CF rational must then be constructed such that the denominator of the CFk term bears UOMi units UOMi 1 1 UOMf 1 UOMf 1 Initial CFk CFf Final = UOMi ④ The CF selected must further satisfy the condition that the UOM of the CFk term numerator will transitively match a corresponding CFf denominator to complete the desired quantity unit conversion UOMi 1 1 UOMf 1 UOMf 1 = UOMi ⑤
- 50. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Unit Factors Method of Dimensional Analysis ( MODA ) Factor Label Method ( FLM ) or Unit Factor Method ( UFM ) Problem Solving Technique UOMi 1 If a CF term satisfying the condition that the UOM of the CFk term numerator transitively match a corresponding CFf denominator term cannot quite be had, yet another CF term will need to be introduced. UOMf 1 UOMf 1 = Initial CFf Final UOMi 1 This added term – as was desired of the two-term CF case – denoted CFn, will best be selected to form a transitive chain with the Initial and Final terms via apposite alteration of UOMs in the numerators and denominators of the chained terms respectively. 1 1 UOMf 1 UOMf 1 Initial CFk CFn CFf Final = ⑥ 1 CFk UOMi Additional CF’s will be inserted here UOMi ⑦
- 51. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Unit Factors Method of Dimensional Analysis ( MODA ) Factor Label Method ( FLM ) or Unit Factor Method ( UFM ) Problem Solving Technique Thus constructed, the transitive chain of CF terms can be extended by further interpolation of CFs to the chain ( denoted by either a CFk + 1 or CFn – 1 incrementation ) to accommodate any length of expression needed to effect the desired UOM transformation Initial CFk CFn CFf Final UOMi 1 1 1 UOMf 1 UOMf 1UOMi = ⑧
- 52. © Art Traynor 2011 Chemistry Dimensional Analysis Unit Factors Method of Dimensional Analysis ( MODA ) Factor Label Method ( FLM ) or Unit Factor Method ( UFM ) AT Problem Solving Technique ( ATPST ) Example: UOMi 1 ① UOMf 1 → We begin by populating the UFM-PST table with the data provided Initial Final A student has entered a 10.0-km run. How long is the run in miles? Section 1.6 (Pg. 19) 10.0-km 1 Lf -mi 1 → Problem Data & Formulae Li = 10.0-km UOMf = mi CFf = mi km
- 53. © Art Traynor 2011 Chemistry Dimensional Analysis Unit Factors Method of Dimensional Analysis ( MODA ) Factor Label Method ( FLM ) or Unit Factor Method ( UFM ) AT Problem Solving Technique ( ATPST ) Example: An equation is constructed, with the Initial term occupying the LHS and the Final term occupying the RHS of the equality. At least one CF will be necessary ( presuming a CF rational featuring UOMf units as dividend and with UOMi units for a divisor can be furnished for the calculation) in which case the Final term is replicated on the RHS as the singular CF = CFf A student has entered a 10.0-km run. How long is the run in miles? Section 1.6 (Pg. 19) Problem Data & Formulae Li = 10.0-km UOMf = mi CFf = ? mi km UOMi 1 UOMf 1 UOMf 1 = Initial CFf Final UOMi 10.0-km 1 Lf -mi 1 Lf -mi 1 = UOMi CF1 = 1km 1000m CF1 is not composed of the necessary UOMs so additional CF rationals will need to be introduced, but it does suggest the reciprocal will advance us toward the solution. mi km ≠ ≠ ②
- 54. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Unit Factors Method of Dimensional Analysis ( MODA ) Factor Label Method ( FLM ) or Unit Factor Method ( UFM ) Problem Solving Technique UOMi 1 Lacking for a singular CF, the additional CF , denoted CFk , will be interpolated, and juxtpositioned between the Initial term and CFf UOMi 1 UOMf 1 UOMf 1 Initial CFk CFf Final ③ = Example: A student has entered a 10.0-km run. How long is the run in miles? Section 1.6 (Pg. 19) Problem Data & Formulae Li = 10.0-km UOMf = mi CFf = ? mi km CF1 = 1km 1,000m mi km 10.0-km 1 UOMi 1 Lf -mi 1 Lf -mi 1 = UOMi -km CF2 = 1m 1.094yd mi km ≠ = ≠ ≠ CF2 fails to supply a CF matching the UOMs needed. This suggests an additional term will need to be interpolated to form a transitive chain (of the form yards-per-mile). 10.0-km 1 UOMi 1 Lf -mi 1 Lf -mi 1 = 10.0-km 1,000-m
- 55. © Art Traynor 2011 Chemistry Dimensional Analysis Unit Factors Method of Dimensional Analysis ( MODA ) Factor Label Method ( FLM ) or Unit Factor Method ( UFM ) Problem Solving Technique This added term – as was desired of the two-term CF case – denoted CFn, will best be selected to form a transitive chain with the Initial and Final terms via apposite alteration of UOMs in the numerators and denominators of the chained terms respectively. Example: A student has entered a 10.0-km run. How long is the run in miles? Problem Data & Formulae Li = 10.0-km UOMf = mi CFf = ? mi km CF1 = 1km 1,000m mi km CF2 = 1m 1.094yd mi km ≠ = ≠ ≠ CF3 supplies the missing UOM (in reciprocal) equivalency we need to complete the transitive chain, which also matches up with the UOM terms in the CFn term 10.0-km 1 1 1 Lf -mi 1 Lf -mi 1 Initial CFk CFn CFf Final = 10.0-km 1,000-m CF3 = 1,760 yd 1mi mi km ≠ ≠ 10.0-km 1 1 1 1-mi 1 Lf -mi 1 = 10.0-km 1,000-m ④ 1-m 1.094-yd 1,760-yd
- 56. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Mathematical Implications Mathematical Implications of DA Dimension The indexed product of a fundamental physical Metric ( e.g. Length, Mass, Time, Charge, Temperature ) by a rational argument is said to constitute the Dimension of that phenomenological equivalence class ( i.e. physical quantity ) Scale Unit The notion of Dimension is hierarchically distinct from the notion of Scale Unit Mass Scale Unit Pound ( lb )Gram ( g ) Dimension ( of Mass ) Scale Units ( in the Mass Dimension ) Any Legth has a Dimension of “L” irrespective of what units of “Length” are selected by which to make a measure ( see Dimensional Invariance )
- 57. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Mathematical Implications Mathematical Implications of DA Dimension The fundamental physical Metrics constituting physical dimensions are further characterized by the following properties: Abelian Group over ℚ Vector Space over ℚ The fundamental physical Metrics are understood to constitute a Basis for this Vector Space n A Change of Basis is similarly effected, as in any other vector space, yielding alternate systems of units ( e.g. whether the unit for Charge is derived from the unit for Current or vice versa ) n
- 58. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Limitations Linear Homogeneity Restriction Constitute Linear Combinations of the Dimensional ( Fundamental ) Scale Units The validity of the Factor Label Method ( FLM ) or Unit Factor Method ( UFM ) is limited to well-formed Conversion Factors ( CF’s ) which: Feature homogenous solution sets ( i.e. with intercepts intersecting the vector space at its coordinate origin ). and Temperature CFs ( in particular ) are problematic because: Celsius ↔ FahrenheitCelsius ↔ Kelvin Constant Difference∆ ∝ In-Constant Ratio In-Constant Difference∆ ∝ In-Constant Ratio Temperature can summarily be defined as a measure of the internal energy present in an phenomenological system
- 59. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Limitations Linear Homogeneity Restriction The validity of the Factor Label Method ( FLM ) or Unit Factor Method ( UFM ) is limited to well-formed Conversion Factors ( CF’s ) which: Temperature CFs ( in particular ) are problematic because: Celsius ↔ FahrenheitCelsius ↔ Kelvin Constant Difference∆ ∝ In-Constant Ratio In-Constant Difference∆ ∝ In-Constant Ratio Conversion Factors ( CF’s ) 100 °C 212 °F – 32 °F °F = A ratio of the scale interval over which water H2O maintains a liquid state 100 °C 180 °F °F = = °C + 32 5 9 °C = °K – 273.15 Conversion Factors ( CF’s ) – 40° C = – 40° F
- 60. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Limitations Linear Homogeneity Restriction The validity of the Factor Label Method ( FLM ) or Unit Factor Method ( UFM ) is limited to well-formed Conversion Factors ( CF’s ) which: Temperature CFs ( in particular ) are problematic because: Celsius ↔ FahrenheitCelsius ↔ Kelvin Constant Difference∆ ∝ In-Constant Ratio In-Constant Difference∆ ∝ In-Constant Ratio Conversion Factors ( CF’s ) 100 °C 212 °F – 32 °F °C = A ratio of the scale interval over which water H2O maintains a liquid state 100 °C 180 °F °C = = ( °F – 32 ) 9 5 °K = °C + 273.15 Conversion Factors ( CF’s ) – 40° C = – 40° F
- 61. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Limitations Linear Homogeneity Restriction Fahrenheit °F The “scaling” of the various standards of Temperature measure accounts for the CF difficulty in applying FLM / UFM After Daniel Gabriel Fahrenheit ( 1686 – 1736 ) , German Physicist Tripartite Reference Interval ( defining unit scale ) Ambient Body Temperature ranks 96°Fn Frigorific Equilibrium of water-ice H2O slurry ranks 32°F n Frigorific Equilibrium of Ammonium Chloride NH4CL ranks 0°F n Danzig Polish-Lithuanian Commonwealth
- 62. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Limitations Linear Homogeneity Restriction Celsius °C The “scaling” of the various standards of Temperature measure accounts for the CF difficulty in applying FLM / UFM After Anders Celsius ( 1701 – 1744 ) , Swedish Astronomer Original Bipartite Reference Interval ( defining unit scale ) Boiling point of water H2O ranks 100°Cn Freezing point of water H2O ranks 0°Cn Grand Principality of Transylvania Contemporary Bipartite Reference Interval Triple Point of water H2O ranks 100°Cn Absolute zero 0°K = – 273.15°Cn o The Triple Point of a substance is that unique temperature and pressure at which the three phases coexist in thermodynamic equilibrium
- 63. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Limitations Linear Homogeneity Restriction Celsius °C The “scaling” of the various standards of Temperature measure accounts for the CF difficulty in applying FLM / UFM After Anders Celsius ( 1701 – 1744 ) , Swedish Astronomer Grand Principality of Transylvania The Celsius and Kelvin temperature scales share the same interval (defined by the partition of the interval between absolute zero 0°K and the Triple Point of water H2O at 273.15 °K , or one part in 273.15, or 0.003661). n Contemporary Bipartite Reference Interval | Ttp – T0 | 1 = I
- 64. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Limitations Linear Homogeneity Restriction Celsius °C The “scaling” of the various standards of Temperature measure accounts for the CF difficulty in applying FLM / UFM After Anders Celsius ( 1701 – 1744 ) , Swedish Astronomer Grand Principality of Transylvania Contemporary Bipartite Reference Interval Interval System of Measure The Celsius system employs a relative scale of measure, as opposed to the absolute scales on which other fundamental scale units are premised 200-ft is twice as long as 100-ft, but 20°C water does not is not twice as thermally energetic as 10°C water n
- 65. © Art Traynor 2011 Chemistry Dimensional Analysis Wiki “Dimensional Analysis” Limitations Linear Homogeneity Restriction Kelvin °K The “scaling” of the various standards of Temperature measure accounts for the CF difficulty in applying FLM / UFM After William Thompson, Lord (Baron) Kelvin ( 1824 – 1907 ) , British Mathematical Physicist Grand Principality of Transylvania United Kingdom of Great Britain & Ireland Interval System of Measure A unit of measure for Temperature premised on a Thermodynamic, or absolute, scale and one of the seven base units of the SI system Coincident with the Celsius scale intervaln o Preserving simplicity by a “ constant difference ” in conversion between the two systems
- 66. © Art Traynor 2011 Chemistry Properties of Substances Density Density : ⍴ (rho) A fundamental property of any substances is its density. Density is the mass per unit volume of any substance There are several related notions ⍴ = → m V mass volume Units are Kilograms per Cubic Meter kg m3
- 67. © Art Traynor 2011 Chemistry Properties of Substances Density Density : ⍴ (rho) A fundamental property of any substances is its density. Density is the mass per unit volume of any substance Specific or Unit Weight The weight per unit volume of a material ⍴ = → in m V mass volumeThere are several related notions Units are Newtons per Cubic Meter N m3 kg m3 m s2 G in γ = ⍴ · G → · G → → m V m · G V w V Can be affected by variations in Temperature and Pressure ( via the Bulk Modulus of the material )
- 68. © Art Traynor 2011 Chemistry Properties of Substances Density Density : ⍴ (rho) A fundamental property of any substances is its density. Density is the mass per unit volume of any substance Specific or Unit Weight The weight per unit volume of a material ⍴ = → in m V mass volumeThere are several related notions kg m3 γ = → in w V weight volume N m3 Relative Density Density expressed as a ratio of one material to a referent material RD = ⍴s ⍴r
- 69. © Art Traynor 2011 Chemistry Properties of Substances Density Density : ⍴ (rho) A fundamental property of any substances is its density. Density is the mass per unit volume of any substance Specific or Unit Weight The weight per unit volume of a material ⍴ = → in m V mass volumeThere are several related notions kg m3 γ = → in w V weight volume N m3 Relative Density Density expressed as a ratio of one material to a referent material Specific Gravity Relative Density expressed as a ratio of a material to water ( the referent material ) RD = ⍴s ⍴r
- 70. © Art Traynor 2011 Chemistry Properties of Substances Density Density : ⍴ (rho) A fundamental property of any substances is its density. Density is the mass per unit volume of any substance Specific or Unit Weight The weight per unit volume of a material ⍴ = → in m V mass volumeThere are several related notions kg m3 γ = → in w V weight volume N m3 RD = ⍴s ⍴r Densities do not necessarily correlate to atomic masses Atomic spacings and crystalline structure affect elemental density Avagadro’s Number Density derived Metrics such as Molar Volume encode Avagadro’s Number and their dependence on atomic masses n
- 71. © Art Traynor 2011 Chemistry Principles Wiki: “ Matter” Structure of Matter Definition of Matter Matter comprises the fundamental ontological constituents of which a phenomenological system is composed, classically reckoned as that which occupies space ( i.e. characterized by volume ) and expresses a mass Properly excludes massless Particles ( e.g. Photon ) Not to be conflated with Mass “Particles” do not necessarily constitute Matter! Matter can assume a continuum of states of aggregation, determined by intrinsic quantifiable parameters such as pressure, temperature, and volume. States / Phases of Matter Solid Liquid Gas Exotics ( Plasma, Super-states, Condensates )
- 72. © Art Traynor 2011 Chemistry Principles Wiki: “ Chemical Substance” Structure of Matter Matter can assume a continuum of states of aggregation, determined by intrinsic quantifiable parameters such as pressure, temperature, and volume. States / Phases of Matter Solid : Characterized by Rigidity Liquid : Characterized by Volumetric Fluidity Gas : Characterized by Volumetric and Morphological Fluidity Fixed Volume Fixed Shape ( Morphology ) Fixed Volume Indeterminate Shape ( Morphology ) Indeterminate Volume Indeterminate Shape ( Morphology )
- 73. © Art Traynor 2011 Chemistry Principles Wiki: “ Matter” Structure of Matter Definition of Matter Matter comprises the fundamental ontological constituents of which a phenomenological system is composed, classically reckoned as that which occupies space ( i.e. characterized by volume ) and expresses a mass Properly excludes massless Particles ( e.g. Photon ) Not to be conflated with Mass “Particles” do not necessarily constitute Matter! Definition of Substance A configuration of Matter featuring: Constant Chemical Composition Characteristic Properties cannot be separated into more elemental components without breaking chemical bonds Not to be conflated with a mixture From which Law of Constant Composition arises Suited to both an Element and a Homogenous “Compound”
- 74. © Art Traynor 2011 Chemistry Principles Structure of Matter A species of Substance : Composed of two or more Elements, characterized by : Definition of Compound Constant Composition / Unique Chemical Structure Resolution into Elements via Chemical Processes Substance criteria Bonded ( Structure ) Fixed ratio of Atoms ( e.g. Formula )n Representation by a Formula
- 75. © Art Traynor 2011 Chemistry Principles Wiki: “ Chemical Element” Structure of Matter Definition of Element Definition of Mixture Elements are those fundamental constituents of matter uniquely characterized by an atomic number, the chemical properties of which obey a periodic verisimilitude ordering distinctive phenomenological classes among its representatives. A mixture is a combination of unbound substances, capable of separation, the constituents of which remain chemically distinct and irreducible. Wiki: “ Mixture”
- 76. © Art Traynor 2011 Chemistry Principles Wiki: “ Chemical Element” Structure of Matter Definition of Element Elements are those fundamental constituents of matter uniquely characterized by an atomic number, the chemical properties of which obey a periodic verisimilitude ordering distinctive phenomenological classes among its representatives. Properties of Elements Atomic Number Proton Cardinalityn An element’s Atomic Number provides the cardinality of the protons in its nucleus Singularity of Atomic Numbern Each element has a unique and singular atomic number exclusively distinguishing it from any other element Aluminium 2 13 26.9815386 GIIIA (13): Post-Transition Metal P3 8 [Ne]3s23p1 3 Metal Al
- 77. © Art Traynor 2011 Chemistry Principles Wiki: “ Chemical Element” Structure of Matter Definition of Element Elements are those fundamental constituents of matter uniquely characterized by an atomic number, the chemical properties of which obey a periodic verisimilitude ordering distinctive phenomenological classes among its representatives. Properties of Elements Aluminium 2 13 26.9815386 GIIIA (13): Post-Transition Metal P3 8 [Ne]3s23p1 3 Metal Al Periodicity Chemical properties of the Elements were observed to recur with regular recurrence, the patterns of which are encoded in the Periodic Table Al13
- 78. © Art Traynor 2011 Chemistry Principles Wiki: “ Chemical Element” Structure of Matter Definition of Element Elements are those fundamental constituents of matter uniquely characterized by an atomic number, the chemical properties of which obey a periodic verisimilitude ordering distinctive phenomenological classes among its representatives. Properties of Elements Aluminium 2 13 26.9815386 GIIIA (13): Post-Transition Metal P3 8 [Ne]3s23p1 3 Metal Al Periodicity The table is superficially organized into Groups ( columns ) and Periods ( rows ) Al13 13 3
- 79. © Art Traynor 2011 Chemistry Principles Wiki: “ Chemical Element” Structure of Matter Definition of Element Elements are those fundamental constituents of matter uniquely characterized by an atomic number, the chemical properties of which obey a periodic verisimilitude ordering distinctive phenomenological classes among its representatives. Properties of Elements Aluminium 2 13 26.9815386 GIIIA (13): Post-Transition Metal P3 8 [Ne]3s23p1 3 Metal Al Periodicity Groups collect Elements with similar chemical properties and exhibit trends in Atomic Radius ( Positively ) and in Electron Affinity and Ionization Energy ( Inversely ) n ① ② ③ 3d ④⑤ ⑥ ⑦⑧⑨ 10 11 12 13 14 16 17 18 15 Al13
- 80. © Art Traynor 2011 Chemistry Principles Wiki: “ Chemical Element” Structure of Matter Definition of Element Elements are those fundamental constituents of matter uniquely characterized by an atomic number, the chemical properties of which obey a periodic verisimilitude ordering distinctive phenomenological classes among its representatives. Properties of Elements Aluminium 2 13 26.9815386 GIIIA (13): Post-Transition Metal P3 8 [Ne]3s23p1 3 Metal Al Periodicity Periods collect Elements with similar electronic structure ( incremented by Atomic Number ) and exhibit trends in Electron Affinity and Ionization Energy ( positively ), and Atomic Radius ( Inversely ) n 1 2 4 5 6 7 Al133
- 81. © Art Traynor 2011 Chemistry Principles Structure of Matter Definition of Mixture A mixture is a combination of unbound substances, capable of separation, the constituents of which remain chemically distinct and irreducible. Wiki: “ Mixture” Properties of Mixtures HeterogenousHomogenous Visually Indistinguishable Constituents N N Absence of Chemical Bonds The main distinguishing characteristic of a mixture is the homogeneity of its composition Visually Distinct Constituents o Solution ( Gaseous /Aqueous /Concretion ) Variable Composition
- 82. © Art Traynor 2011 Chemistry Principles Fundamental Laws of Chemistry Law of Conservation of Mass ( LOCOM ) For a phenomenological system, closed to extraneous transfers of Matter & Energy, the Mass of the system must remain constant over time, implying: Mass can neither be created or destroyed Section 2.1 (Pg. 44) Mass can only be spatially rearranged Law of Definite Proportion ( LODP ) A chemical compound always contains exactly the same proportion of Elements by Mass Section 2.1 (Pg. 44)
- 83. © Art Traynor 2011 Chemistry Principles Fundamental Laws of Chemistry Conservation of Mass For a phenomenological system, closed to extraneous transfers of Matter & Energy, the Mass of the system must remain constant over time, implying: Mass can neither be created or destroyed Section 2.1 (Pg. 44) Mass can only be spatially rearranged Definite Proportion A chemical compound always contains exactly the same proportion of Elements by Mass Section 2.1 (Pg. 44) Multiple Proportions For two Elements capable of forming at least two distinct Compounds the ratios of the Masses of the second element combining with one gram of the first will always reduce to a small whole number Section 2.1 (Pg. 44)
- 84. © Art Traynor 2011 Chemistry Atomic Theory Historical Development Dalton’s “ New System of Chemical Philosophy ” Each element is composed of miniscule particles or Atoms Section 2.3 (Pg. 46) The Atoms of a Element are identical United KingdomCounty of Cumbria 1766 – 1844 JohnDalton Compounds are composed of disparate Elements and always expresses the same relatives numbers and species of Atoms Reactions entail the reorganization of Atoms ( in the manner in which they are bound ) and remain unaffected fundamentally by a reaction
- 85. © Art Traynor 2011 Chemistry Atomic Theory Historical Development Avogadro’s Hypothesis At constant/equal Temperature and Pressure: Section 2.3 (Pg. 46) Kingdom of SardiniaCity of Turin 1776 – 1856 AmadeoAvogadro Refining the volumetric gas reactant results of Joseph Gay-Lussac , Avogadro postulated that gasses combine – irrespective of molecular size – in equal volumes and thus equal particulate cardinality Equal volumes of disparate ( reactant ) gasses contain the same number of particles Implying the sizes of the particles are negligible compared to the distances separating constituent particles
- 86. © Art Traynor 2011 Chemistry Atomic Theory Historical Development Thomson’s Cathode Ray Particles ( Electrons ) The particles exhibit directionality – they emanate at the negative electrode ( i.e. Cathode ) and terminate at the positive ( i.e. Anode ) Section 2.3 (Pg. 50) United KingdomCounty of Lincolnshire 1856 – 1940 Joseph John ( J.J. ) Thomson High voltage applied to an evacuated tube produces a stream of particles between electrodes observed to possess the following properties: The particle stream ( i.e. Cathode Rays ) are repelled by the negative pole of an applied electric field. First observed in 1869 by German physicist Johann Hittorf, named in 1876 by Eugen Goldstein Kathodenstrahlen ( i.e. “ Cathode ” ) The Charge-to-Mass ratio of the constituent particles in the stream can be determined by a measurement of deflection of the stream subject to a magnetic field : charge mass = = 1.76 x 108 C/g e m Electrodes of various metallic composition were each observed to produce Cathode Rays , implying that all atoms must contain the negative charge particles thus designated “ Electrons ”
- 87. © Art Traynor 2011 Chemistry Atomic Theory Historical Development Thomson’s Plum Pudding Atomic Model As the Atom exhibited electrical ( charge ) neutrality , the Atom must be composed of a particle with opposite and equal charge as the Electron Section 2.3 (Pg. 50) United KingdomCounty of Lincolnshire 1856 – 1940 Joseph John ( J.J. ) Thomson Thomson’s description of the Electron lead to a compositional conjecture concerning the structure of the Atom Thomson further speculated that this positive charge would be randomly distributed throughout the Atom with the negatively charged Electrons embedded within the atomic composition ( i.e. a ‘Plum Pudding’ of heterogenous particles ) The Plum Pudding analogy was first proposed by Lord Baron Kelvin, William Thomson ( unrelated )
- 88. © Art Traynor 2011 Chemistry Atomic Theory Historical Development Millikan’s Derivation of the Electron Mass The Oil Drop experiment allowed Millikan to arrive at a precise value for the magnitude of the Electron Unit Charge Section 2.3 (Pg. 51) United StatesState of Illinois 1868 – 1953 Robert Andrews Millikan Millikan contrived an experiment whereby the free-fall of charged oil droplets within an atmospherically controlled chamber could be halted ( equalized to the force of G ) by the application of a voltage across two oppositely charged plates The Plum Pudding analogy was first proposed by Lord Baron Kelvin, William Thomson ( unrelated ) Applying this Electron Unit Charge magnitude to J.J. Thomson’s Charge-to-Mass Ratio allowed Millikan to posit a precise Mass for the Electron at 9.11 x 10 – 31 kg
- 89. © Art Traynor 2011 Chemistry Atomic Theory Historical Development Becquerel’s Discovery of Radiation Becquerel’s results subsequently led to the tri-partite radiation emission classifications : Section 2.3 (Pg. 52) Republic of France Historical Province of Brittany 1852 – 1908 Antoine Henri Becquerel Becquerel serendipitously noted that a concealed Uranium mineral was able to impart an image to an unexposed photographic plate Region of Pays de la Loire Department of Loire-Atlantique αParticle Carries a 2+ Charge ( opposite & twice the Electron )n 7300 times the Mass of the Electron !n β Particle A high-speed Electronn γ Ray A form of high-energy “ light ”n
- 90. © Art Traynor 2011 Chemistry Atomic Theory Historical Development Rutherford’s Characterization of the Nucleus A source utilizing Becquerel’s alpha particles Section 2.3 (Pg. 52) 1871 – 1937 Earnest Rutherford Rutherford devised an experiment to test Thomson’s Plum Pudding model of the Atom whereby : αParticle Carries a 2+ Charge ( opposite & twice the Electron )n 7300 times the Mass of the Electron !n Realm of New Zealand Is trained on a gold foil target to probe the structure of the atom by dispersion of the source in collision/deflection with the target Occasional , significant deflection of the source – through large angles – led to the conclusion that the Atom must be composed of a concentrated , positive-charge massive “ nucleus ” Minimal deflection of the majority of the source moreover led to the added conjecture that the atom must be composed mostly of “ open space ”
- 91. © Art Traynor 2011 Chemistry Atomic Structure Modern Understanding The Modern View of Atomic Structure Nuclear Diameter : ~ 10 – 13 cm Section 2.5 (Pg. 53) Atomic Diameter : ~ 10 – 8 cm The margin at which the electrons are observed to circulate the nucleus Atomic Nucleus Protons Positive Chargen Charge Equal in Magnitude to the Electron’s Negative Chargen Neutrons No Charge ( Neutral )n Same ( essentially ) Mass as Protonn Nuclear Size is minimal compared to Atomic Radii Nuclear Density accounts for most all the Mass of the Atom The Electrons constitute the vast majority of the Atomic Volume
- 92. © Art Traynor 2011 Chemistry Atomic Structure Atomic Particles Atomic Particles Section 2.5 (Pg. 53) Electron 9.11 x 10 – 31 kg 1– The Atomic Constituent Particles Proton 1.67 x 10 – 27 kg 1+ Neutron 1.67 x 10 – 27 kg 0 ( 1.60 x 10 – 19 C ) ( 1.60 x 10 – 19 C ) Particle Mass Charge
- 93. © Art Traynor 2011 Chemistry Atomic Structure Atomic Variants Isotope Section 2.5 (Pg. 54) A Chemical Element variant in which the cardinality of its Neutron constituents differs from its Atomic Number ( e. g. its Proton content which crucially imparts its unique identity as a distinct Element). All isotopes of an Element bear the same Atomic Number All isotopes of an Element share the same number of Protons All isotopes of an Element share the same number of Protons but a differing number of Neutrons From the Greek “ isos ” meaning “ equal ” and “ topos ” meaning “ place ” evoking the notion that Isotopes occupy the “ same place” in the Periodic Table hierarchy A Nucleon is a constituent of the atomic nucleus of which there are two classes: Protons and Neutrons An atom’s Mass Number reflects the cardinality of its Nucleon constituents Wiki: “ Isotope”
- 94. © Art Traynor 2011 Chemistry Atomic Structure Atomic Variants Isotope Section 2.5 (Pg. 54) A Chemical Element variant in which the cardinality of its Neutron constituents differs from its Atomic Number All isotopes of an Element bear the same Atomic Number From the Greek “ isos ” meaning “ equal ” and “ topos ” meaning “ place ” evoking the notion that Isotopes occupy the “ same place” in the Periodic Table hierarchy An atom’s Mass Number ( integer value ) reflects the cardinality of its Nucleon constituents Al13 27 Al13 27 Mass Number Atomic Number → The difference of the Mass Number and the Atomic Number yields the cardinality of the Neutron constituent of the Atom ( or Isotopic form of the Atom ) or the Neutron Number Wiki: “ Isotope”
- 95. © Art Traynor 2011 Chemistry Atomic Combinations Molecules Molecules Section 2.5 (Pg. 55) An Electrically Neutral composition of two or more Atoms adhered by a Chemical Bond Wiki: “ Molecule” Distinguished from Ions by the absence of net Charge By convention Polyatomic Ions are sometimes nevertheless referred to as Molecules Upper Level Equivalence Classifications Homonuclear A molecule composed of two or more Atoms of the same Element ( e.g. a Diatomic Molecule ) Heteronuclear A molecule composed of two or more Atoms of the different Elements
- 96. © Art Traynor 2011 Chemistry Molecular Representation Section 2.5 (Pg. 55) Wiki: “ Chemical Formula” Chemical Formula There are several conventional models and representations by which the Elemental constituents of a Molecule can be represented : Atomic Combinations Molecules Structural Formula Space-Filling Models Ball & Stick Models
- 97. © Art Traynor 2011 Chemistry Molecular Representation Section 2.5 (Pg. 55) Wiki: “ Chemical Formula” Chemical Formula There are several conventional models and representations by which the Elemental constituents of a Molecule can be represented : Atomic Combinations Molecules A symbolic Molecular representation composed of the following: Alpha characters indicating the constituent Elements of the Molecule ( 1 – 3 characters ) Numeric subscripts indicate the cardinality of individual Elements in the molecular composition ( after the fashion of a multiplicative factor ) Polyatomic Ionic constituents are further demarcated by parenthetical inclusion, with like subscription indicating multiplicity ( as with Elemental molecular constituents ) n Ionic constituents may be further denoted by an explicit ( superscripted ) Charge designation ( + / – ) , where a multiplicity of unit charge is indicated by an integer coefficient ( if greater than unity )
- 98. © Art Traynor 2011 Chemistry Molecular Representation Section 2.5 (Pg. 55) Wiki: “ Chemical Formula” Chemical Formula There are several conventional models and representations by which the Elemental constituents of a Molecule can be represented : Atomic Combinations Molecules Structural Formula A Molecular representation composed of the symbolism of the Chemical Formula ( and multiplicity conventions ) supplemented by Bond and Spatial Orientation representations : Dashed lines portray 3D receding molecular constituents whereas graduated wedges portray 3D orientation projecting from a 2D representational surface
- 99. © Art Traynor 2011 Chemistry Molecules Bonding Chemical Bond Section 2.5 (Pg. 55) An attraction between Atoms enabling the formation of Chemical Substances composed of two or more Atoms The electrons and nuclei constituting the Substance formed The Bond originates in an electrostatic force of attraction resulting from: A Dipole moment among the Bonded Substance Wiki: “ Chemical Bond” Strong Bonds Bonds can also be characterized by their relative strength Covalent Bondingn Ionic Bondingn Weak Bonds Dipole-Dipole Interactionsn London Dispersion Forcen Hydrogen Bondingn
- 100. © Art Traynor 2011 Chemistry Molecules Bonding Chemical Bonds - Classified Section 2.5 (Pg. 55) Wiki: “ Covalent Bond” Strong Bonds Bonds can also be characterized by their relative strength Covalent Bondingn A Bond fashioned by the sharing of Electrons as between the constituent Elements of a Molecule o Only a distinct number of Electrons participate in the Bond and are thus designated the Shared Pairs or Bonding Pairs o Covalent Bonding can proceed to the extent that each Atom in the Molecular composition is considered to have thus attained a full outer shell corresponding to a stable ( energetically minimized ) Electronic configuration
- 101. © Art Traynor 2011 Chemistry Chemical Bonds - Classified Section 2.5 (Pg. 55) Wiki: “ Covalent Bond” Strong Bonds Bonds can also be characterized by their relative strength Ionic Bonding ( Electrovalence )n A Bond fashioned by the electrostatic attraction as between oppositely charged Ions among the constituent Elements of a Molecule o An Ion with electron cardinality less than its Elemental Atomic Number – a Cation – will exhibit a positive charge, the magnitude of which is an integer multiple of product of the unit Charge and the difference between its Atomic Number and electron cardinality Atomic Combinations Molecules o An Ion with electron cardinality greater than its Elemental Atomic Number – a Anion – will exhibit a negative charge, the magnitude of which is an integer multiple of product of the unit Charge and the difference between its electron cardinality and Atomic Number
- 102. © Art Traynor 2011 Chemistry Chemical Bonds - Classified Section 2.5 (Pg. 55) Wiki: “ Covalent Bond” Strong Bonds Bonds can also be characterized by their relative strength Ionic Bonding ( Electrovalence )n A Bond fashioned by the electrostatic attraction as between oppositely charged Ions among the constituent Elements of a Molecule Typical interaction/product species include: o A metal and non-metal wherein a net transfer of electrons proceeds to such extent that both atoms achieve a state wherein their valence shells can be considered to be simultaneously filled Atomic Combinations Molecules o Reactants wherein a relatively large difference in Electronegativity exists between constituent species o Salts
- 103. © Art Traynor 2011 Chemistry Chemical Bonds - Classified Section 2.5 (Pg. 55) Wiki: “ Covalent Bond” Strong Bonds Bonds can also be characterized by their relative strength Ionic Bonding ( Electrovalence )n A Bond fashioned by the electrostatic attraction as between oppositely charged Ions among the constituent Elements of a Molecule Typical interaction species can be characterized by: o Electrical conductivity ( in solution or molten state ) Atomic Combinations Molecules o Aqueous solubility
- 104. © Art Traynor 2011 Chemistry Structure of Matter Wiki: “ Chemical Element” Periodicity Definition of Element Elements are those fundamental constituents of matter uniquely characterized by an atomic number, the chemical properties of which obey a periodic verisimilitude ordering distinctive phenomenological classes among its representatives. Properties of Elements Periodicity: Chemical properties of the Elements were observed to recur with regular recurrence, the patterns of which are encoded in the Periodic Table Upper Level Equivalence Classifications Metals Non-Metals
- 105. © Art Traynor 2011 Chemistry Structure of Matter Wiki: “ Chemical Element” Periodicity Properties of Metals Ready Conductors of Heat & Electricity Malleable ( can be formed into thin sheets ) Ductile ( can be formed into wires ) Lustrous appearance Electron Donors forming Positive Ions ( typically ) Properties of Non-Metals Tend to form Diatomic ( Homonucelar ) Molecules Reactions with metals tend to produce Ionic Salts Electron Receptors forming Negative Ions ( typically )
- 106. © Art Traynor 2011 Chemistry Structure of Matter Periodicity Families of Elements ( Groups ) The Elements may be further classified according to representative “ families ” exhibiting similar chemical properties. These class-similar elements are arrayed within the Periodic Table vertically into Groups Second Level Equivalence Classifications Alkali Metals ( Group 1 ) Section 2.7 (Pg. 60) Hydrogen, Lithium, Sodium, Potassium, Cesium, Francium Tend to form 1+ Ions when reacted with non-metalsn ① H1 Na11 Li3 K19 Rb37 Cs55 Fr87
- 107. © Art Traynor 2011 Chemistry Structure of Matter Periodicity Families of Elements ( Groups ) The Elements may be further classified according to representative “ families ” exhibiting similar chemical properties. These class-similar elements are arrayed within the Periodic Table vertically into Groups Second Level Equivalence Classifications Alkaline Earth Metals ( Group 2 ) Section 2.7 (Pg. 60) Beryllium, Magnesium, Calcium, Strontium, Barium, Radium Tend to form 2+ Ions when reacted with non-metalsn Mg12 Be4 Ca20 Sr38 Ba56 Ra88 ②
- 108. © Art Traynor 2011 Chemistry Structure of Matter Periodicity Families of Elements ( Groups ) The Elements may be further classified according to representative “ families ” exhibiting similar chemical properties. These class-similar elements are arrayed within the Periodic Table vertically into Groups Section 2.7 (Pg. 60) Second Level Equivalence Classifications Halogens ( Group 17 ) Fluorine, Chlorine, Bromine, Iodine, Astatine, Ununseptium Tend to form 1 – Ionic Salts when reacted with metalsn 17 F9 Uus117 I53 Br35 At85 Cl17
- 109. © Art Traynor 2011 Chemistry Structure of Matter Periodicity Families of Elements ( Groups ) The Elements may be further classified according to representative “ families ” exhibiting similar chemical properties. These class-similar elements are arrayed within the Periodic Table vertically into Groups Section 2.7 (Pg. 60) Second Level Equivalence Classifications Nobel Gases ( Group 18 ) Helium, Neon, Argon, Krypton, Xenon, Radon, Ununoctium Tend to manifest as gases with little propensity for Chemical Reactionn 18 Uuo118 Ar18 Ne10 Kr36 Xe54 Rn86 He2